Double Replacement Reaction Worksheet Double replacement reactions also called double displacement exchange or metathesis reactions occur when parts of two ionic compounds are exchanged making two new compounds The overall pattern of a double replacement reaction looks like this A B C D A D C B
A double replacement reaction is a reaction in which the positive and negative ions of two ionic compounds exchange places to form two new compounds The general form of a double replacement also called double displacement reaction is ce AB ce CD rightarrow ce AD ce CB nonumber A double replacement reaction has the form AB CD AD CB There are four different possible outcomes to a reaction such as this 1 Formation of a gas There are certain compounds which are unstable and decompose to water and a gas Three common ones are H2CO3 H2SO3 and NH4OH They decompose like this
Double Replacement Reaction Worksheet

Double Replacement Reaction Worksheet
https://online.fliphtml5.com/mlzt/kjlu/files/large/3.jpg
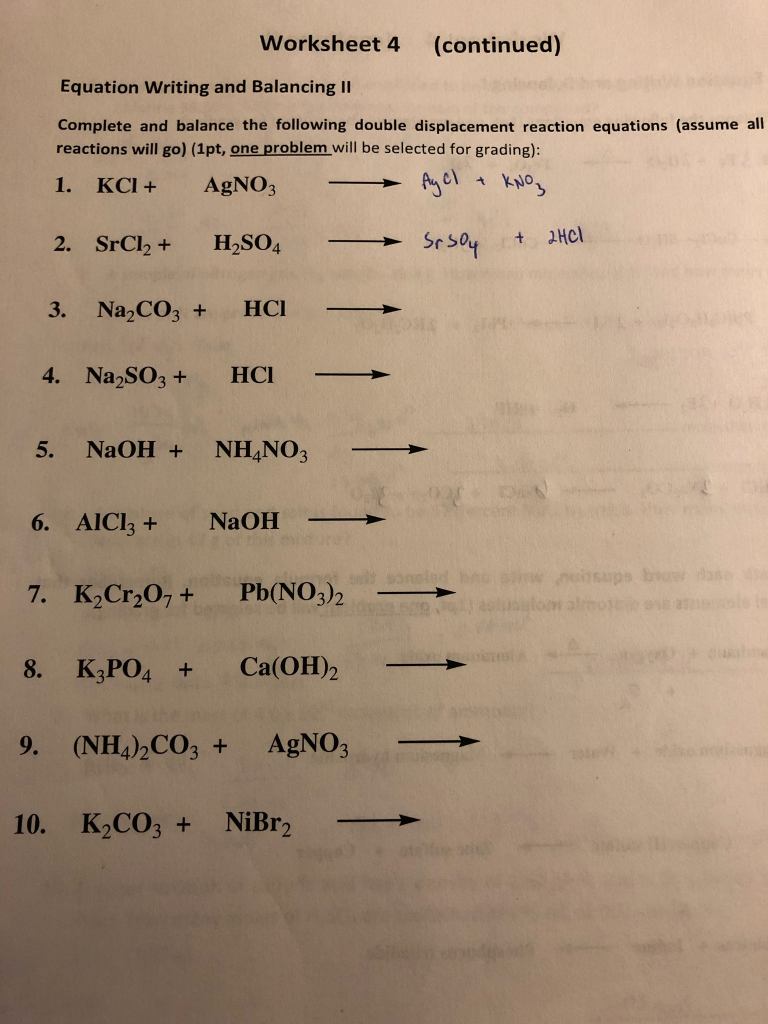
Double Replacement Reaction Worksheet
https://media.cheggcdn.com/media/2a9/2a9b7c30-0a8a-4ca8-9b50-ccd127990f83/php0JSujg.png
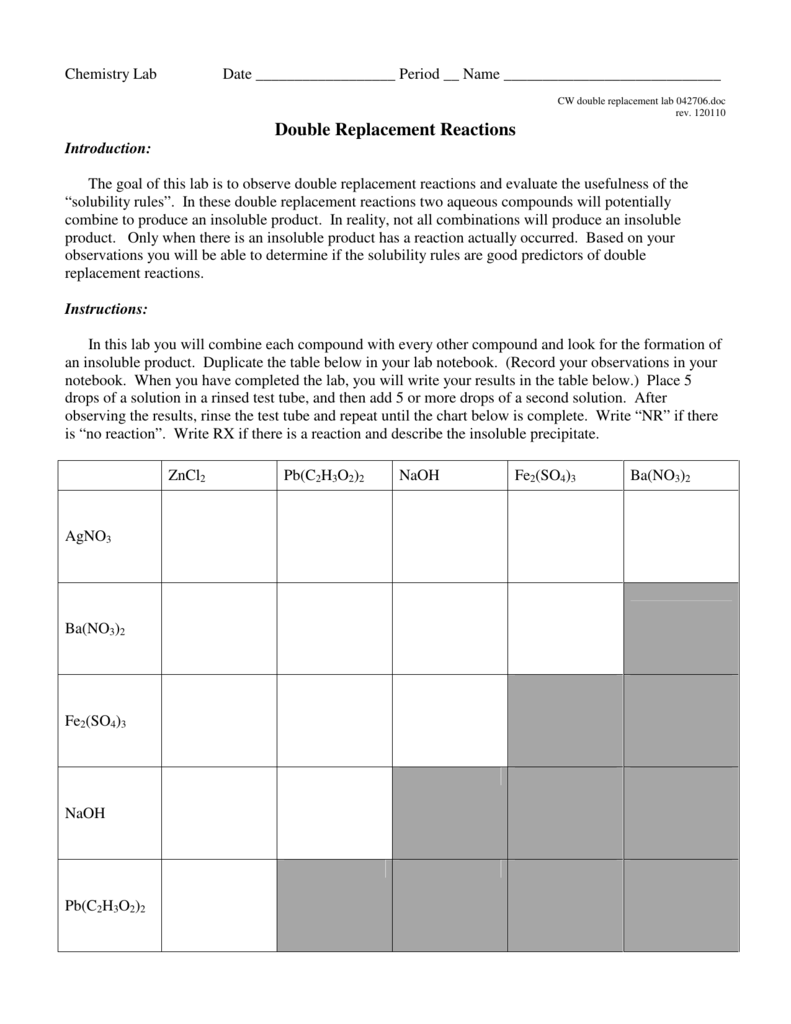
Double Replacement Worksheet Chart Sheet Gallery
https://s3.studylib.net/store/data/008710238_1-68010656b9b765fd945138b1c4bfde68.png
Key Takeaways A single replacement reaction replaces one element for another in a compound The periodic table or an activity series can help predict whether single replacement reactions occur A double replacement reaction exchanges the cations or the anions of two ionic compounds A precipitation reaction is a double replacement reaction When a double replacement reaction occurs the cations and anions switch partners resulting in the formation of two new ionic compounds AD and CB one of which is in the solid state This solid product is an insoluble ionic compound called a precipitate To determine whether a product ionic compound will be soluble or insoluble consult the
CHM 130LL Double Replacement Reactions One of the main purposes of chemistry is to transform one set of chemicals the reactants into another set of chemicals the products via a chemical reaction Reactants Products Double Displacement Reactions Solutions Indicate which of the following double displacement reactions will go to completion and circle what the product of the reaction will be if any You may want to consult a list of Ksp values to solve these problems 1 2 NaOH 1 CaBr2 2 NaBr 1 Ca OH 2 Will this reaction occur Yes 2 1 Pb NO3 2
More picture related to Double Replacement Reaction Worksheet

50 Double Replacement Reaction Worksheet
https://chessmuseum.org/wp-content/uploads/2019/10/double-replacement-reaction-worksheet-inspirational-double-replacement-lab-of-double-replacement-reaction-worksheet.png
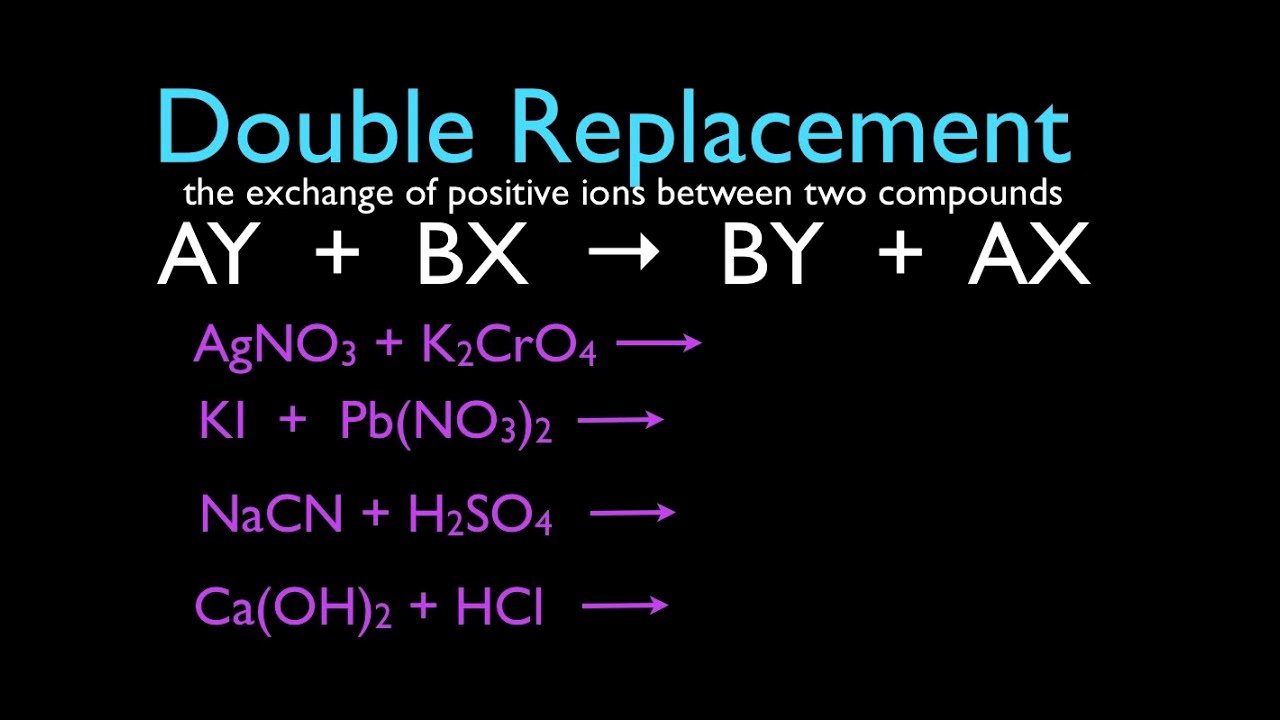
50 Double Replacement Reaction Worksheet
https://chessmuseum.org/wp-content/uploads/2019/10/double-replacement-reaction-worksheet-beautiful-double-replacement-reactions-of-double-replacement-reaction-worksheet.jpg
Double Replacement Reaction Worksheet
https://imgv2-2-f.scribdassets.com/img/document/294765018/original/191f6c5ffc/1627492415?v=1
Objectives Predict if a reaction will occur for double displacement rxns Predict the products of double displacement rxns Predict if these reactions will occur If yes complete and balance the equation You will need to use the Solubility Chart found in your book Double Replacement Reactions H2SO4 aq NaOH aq A double replacement reaction also known as a double displacement reaction is a type of chemical reaction in which two reactants exchange ions to form two new compounds Double replacement reaction takes place in an aqueous solution and typically results in the formation of a precipitate Ionic compounds mostly participate in a double
CHEMISTRY DOUBLE REPLACEMENT REACTION WORKSHEET AgNO3 NaCl AgCl NaNO3 CaCO3 HCl CaCl2 CO2 H2O 7 Pb NO3 2 CuSO4 PbSO4 Cu NO3 2 1 It is important that the formulas of the products be written correctly If they are correct balancing the equation is a simple task if not the equation will Back to Double Replacement Write correct formulas for the products in these double replacement reactions 1 Ca OH 2 H 3 PO 4 Ca 3 PO 4 2 H 2 O
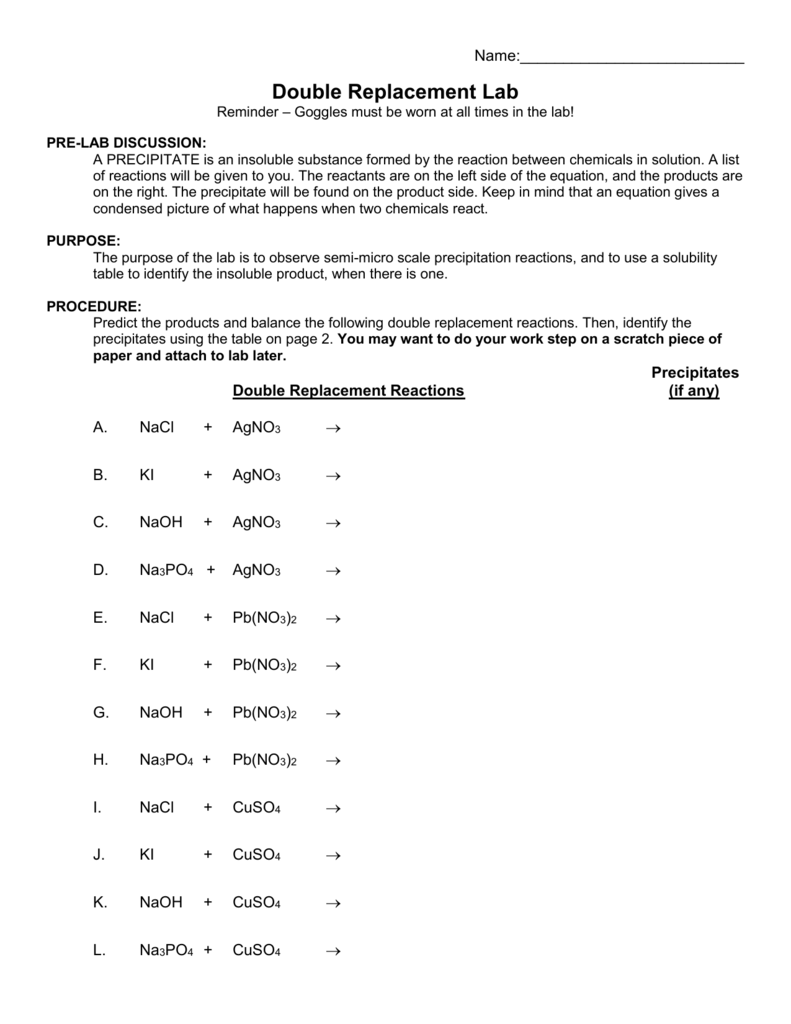
Double Replacement Reaction Worksheet
https://s3.studylib.net/store/data/007019323_1-48844cbdaae7224f907e95d338191424.png

Double Replacement Reaction Worksheet
https://s3.studylib.net/store/data/008629482_1-9a4699a165c11885379b6c0915f2e263.png
Double Replacement Reaction Worksheet - When a double replacement reaction occurs the cations and anions switch partners resulting in the formation of two new ionic compounds AD and CB one of which is in the solid state This solid product is an insoluble ionic compound called a precipitate To determine whether a product ionic compound will be soluble or insoluble consult the
