Double Replacement Reaction Worksheet Answers Back to Double Replacement Write correct formulas for the products in these double replacement reactions 1 Ca OH 2 H 3 PO 4 Ca 3 PO 4 2 H 2 O
In double replacement both reactants are compounds each with a cation part and an anion part Diatomic elements do not count they are included in the single replacement category Here are several examples which are solved below 2 Pair up each cation with the anion from the OTHER compound Double replacement reactions also called double displacement exchange or metathesis reactions occur when parts of two ionic compounds are exchanged making two new compounds The overall pattern of a double replacement reaction looks like this A B C D A D C B
Double Replacement Reaction Worksheet Answers

Double Replacement Reaction Worksheet Answers
https://i.pinimg.com/originals/4a/85/fc/4a85fc14bebd8bff07f09ea722fed418.png
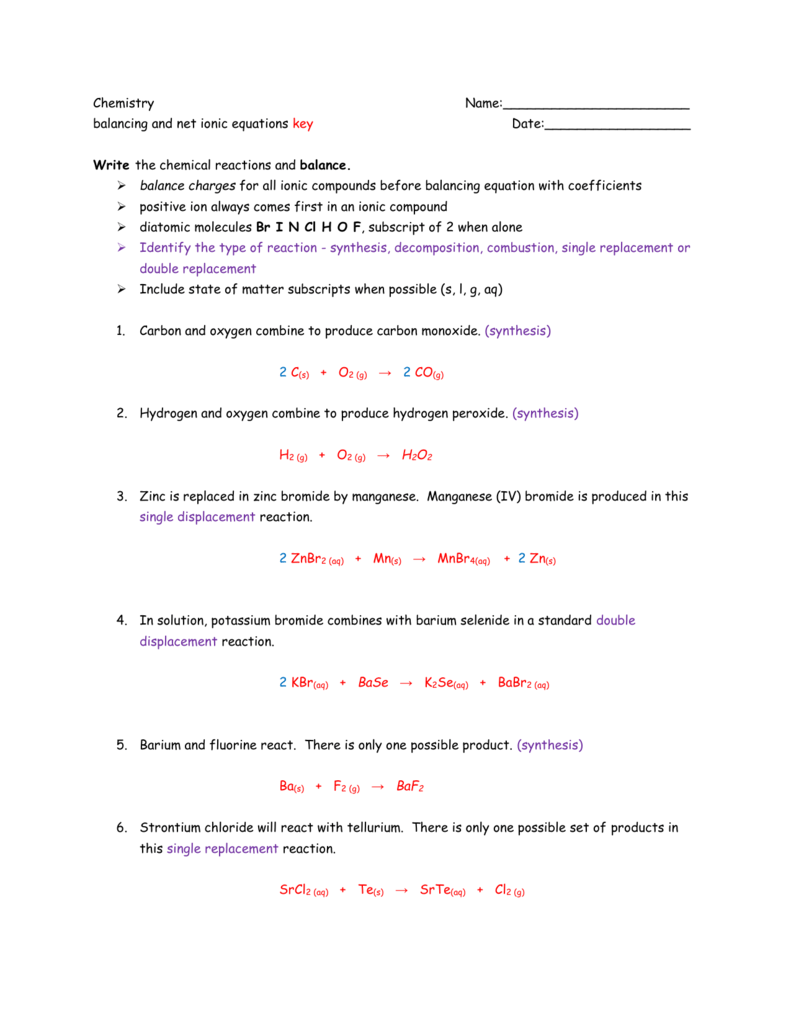
Double Replacement Reactions Worksheet Answer Key 28 Double
https://s3.studylib.net/store/data/007130368_1-56af4f7ae60ae152e3d0dd193eedf922.png

Single And Double Replacement Reactions Worksheets
https://i2.wp.com/s3.studylib.net/store/data/008707859_1-60593c49c27765a0ba6c43886cca4f32.png
WORKSHEET ON SINGLE DOUBLE REPLACEMENT REACTIONS Predict the products Write formulas balance each reaction If there is no reaction then just put NO RXN Single Replacement A BC B AC or A BC C BA when A and C are negative ions Zinc Hydrogen chloride Magnesium Hydrogen Sulfate Copper II chloride Flourine In a precipitation reaction one type of double replacement reaction two soluble ionic compounds in aqueous solution are mixed and result in an insoluble solid compound called a precipitate For example consider the reaction between aqueous lead II nitrate with aqueous potassium bromide as shown below Pb NO3 2 aq KBr aq
When a double replacement reaction occurs the cations and anions switch partners resulting in the formation of two new ionic compounds AD and CB one of which is in the solid state This solid product is an insoluble ionic compound called a precipitate To determine whether a product ionic compound will be soluble or insoluble consult the This worksheet is designed to help you predict products of simple reactions of the four basic reaction types synthesis decomposition single replacement and double replacement and combustion reactions For the first few reactions the type of reaction is listed you should predict the products then balance
More picture related to Double Replacement Reaction Worksheet Answers
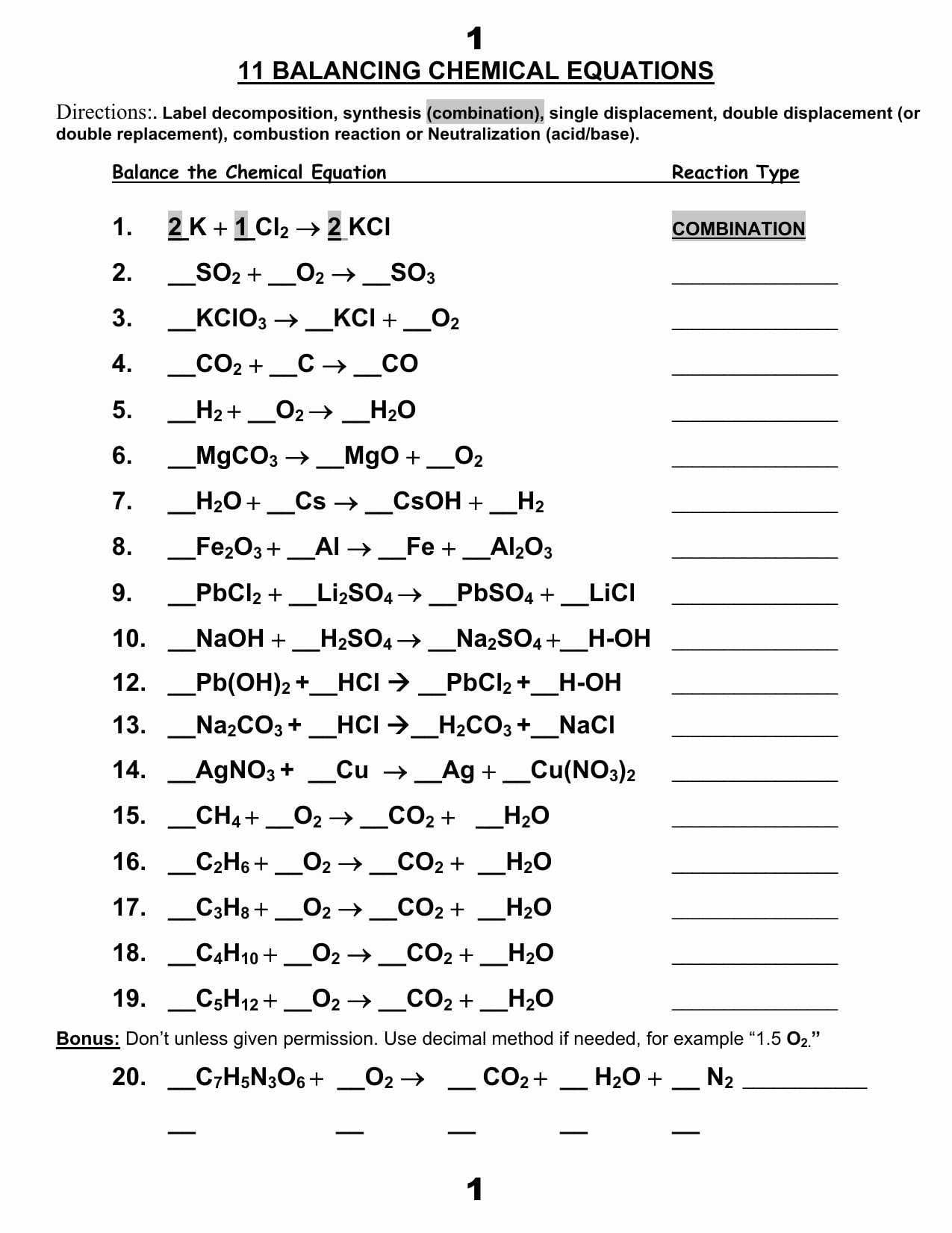
Synthesis And Decomposition Reactions Worksheet Answers Db excel
https://db-excel.com/wp-content/uploads/2019/09/synthesis-and-decomposition-reactions-worksheet-answers-2.jpg
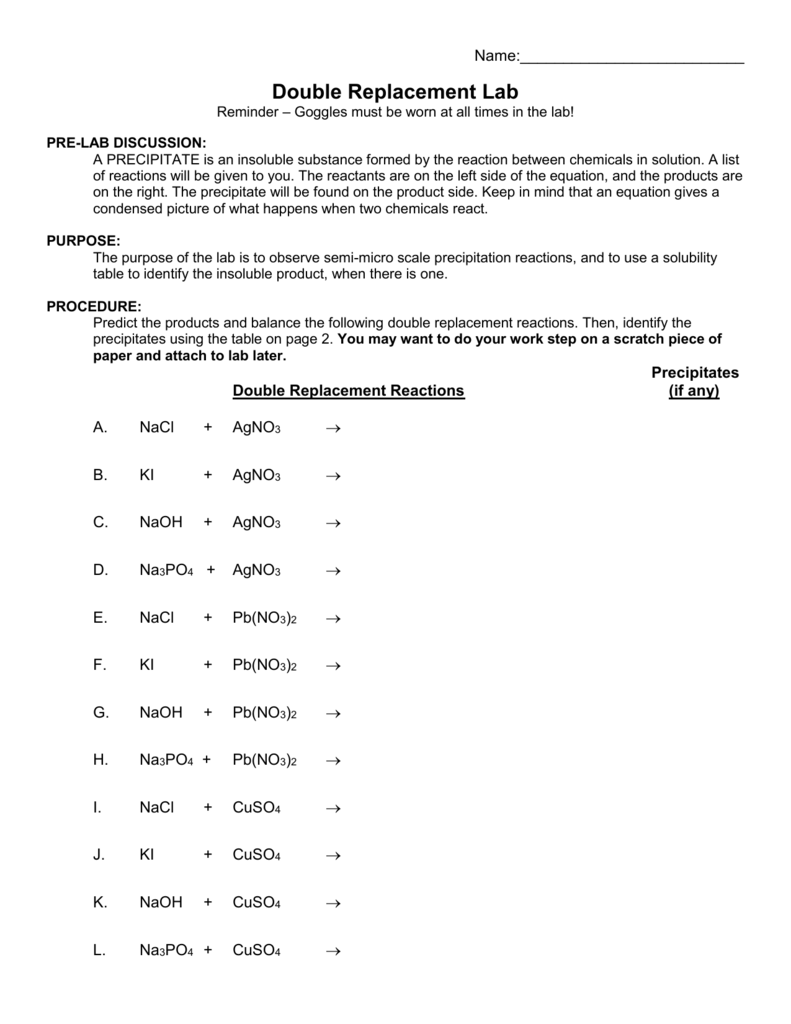
Double Replacement Reaction Worksheet
https://s3.studylib.net/store/data/007019323_1-48844cbdaae7224f907e95d338191424.png
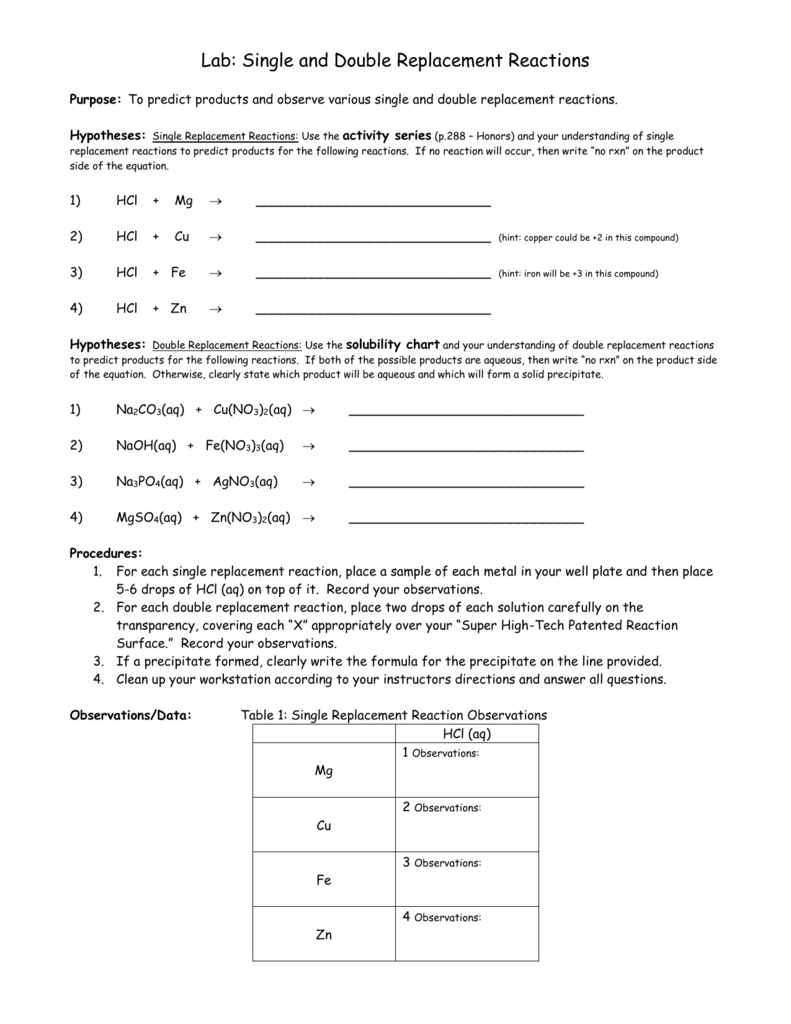
Double Replacement Reactions Worksheet Answer Key 28 Double
https://s3.studylib.net/store/data/008558336_1-560ee40d085e111c61f159975aaf68d8.png
A double replacement reaction is a reaction in which the positive and negative ions of two ionic compounds exchange places to form two new compounds The general form of a double replacement also called double displacement reaction is ce AB ce CD rightarrow ce AD ce CB nonumber A double replacement reaction has the form AB CD AD CB There are four different possible outcomes to a reaction such as this 1 Formation of a gas There are certain compounds which are unstable and decompose to water and a gas Three common ones are H2CO3 H2SO3 and NH4OH They decompose like this
A single replacement reaction is a chemical reaction in which one element is substituted for another element in a compound generating a new element and a new compound as products Presented below 2HCl aq Zn s ZnCl2 aq H2 g 2 HCl aq Zn s ZnCl 2 aq H 2 g is an example of a single replacement reaction Transcribed image text Worksheet 5 Double Replacement Reactions In these reactions all you do is look at the names of the reactants and switch partners Just be sure that the new pairs come out with the positive ion named first and paired with a negative ion 1 alinum iodide mercury I chloride 2 silver nitrate potassium phosphate
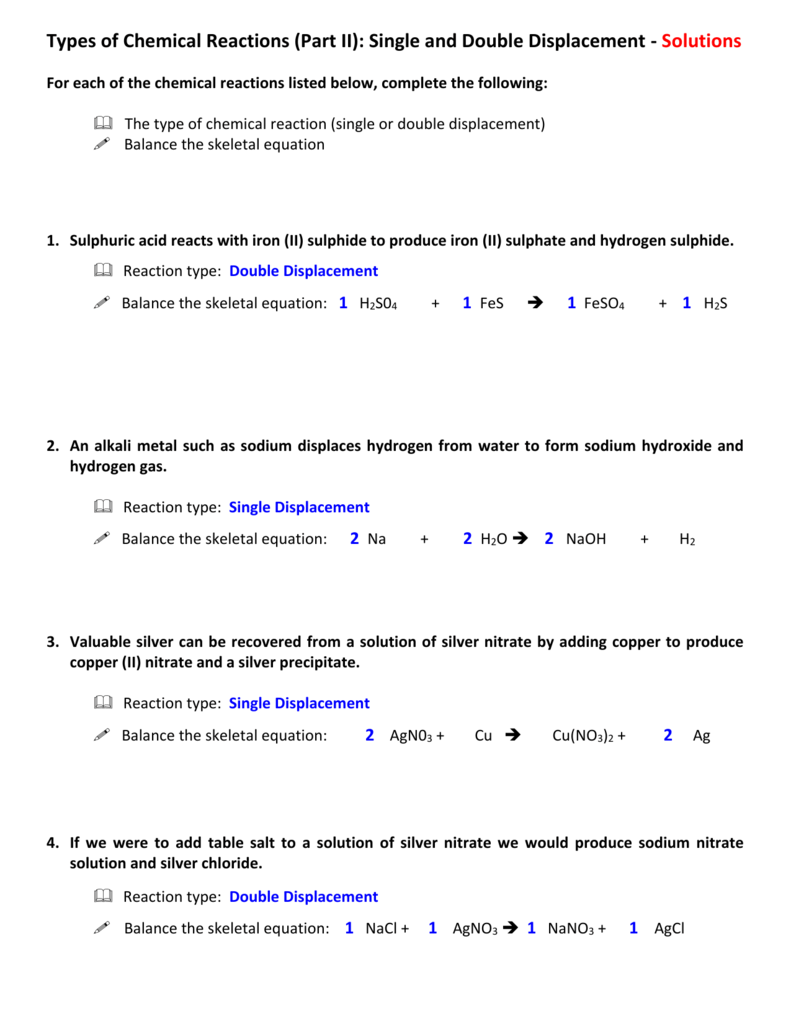
Chemistry Double Replacement Reaction Worksheet Practice Reactions
https://s3.studylib.net/store/data/008553830_1-b0756338a83a1275426b5e20fc64d0fb.png
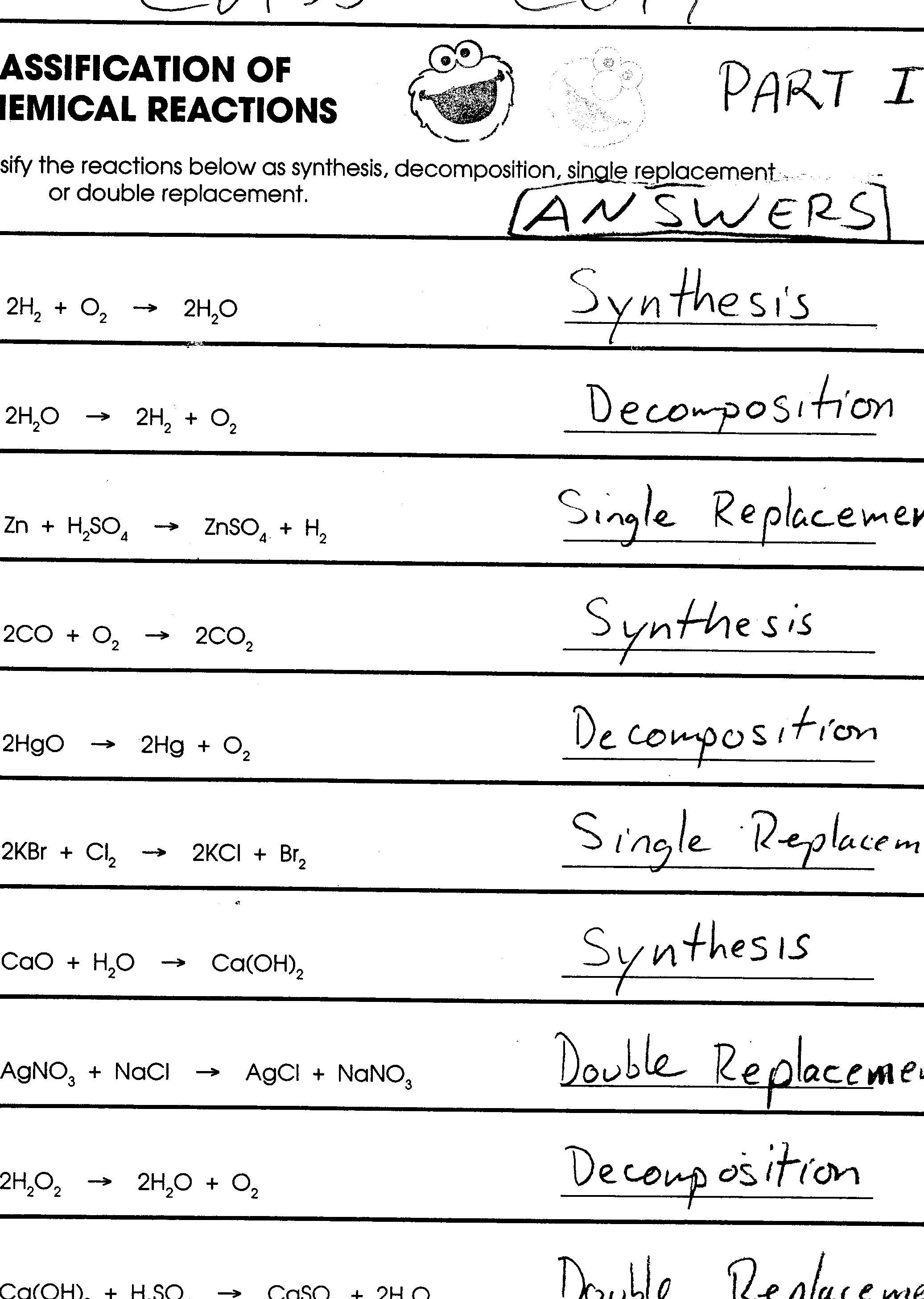
10 Types Of Reactions Worksheet Worksheeto
https://www.worksheeto.com/postpic/2011/07/classifying-chemical-reactions-worksheet-answer-key_278921.jpg
Double Replacement Reaction Worksheet Answers - Double Replacement Reactions Worksheet Objectives Predict if a reaction will occur for double displacement rxns Predict the products of double displacement rxns Predict if these reactions will occur If yes complete and balance the equation You will need to use the Solubility Chart found in your book Double Replacement Reactions 1 H 2 SO 4