Decomposition And Synthesis Reactions Worksheet In decomposition reactions one compound will break down into two or more parts barium carbonate magnesium carbonate potassium carbonate zinc hydroxide Iron II hydroxide nickel II chlorate sodium chlorate potassium chlorate sulfuric acid
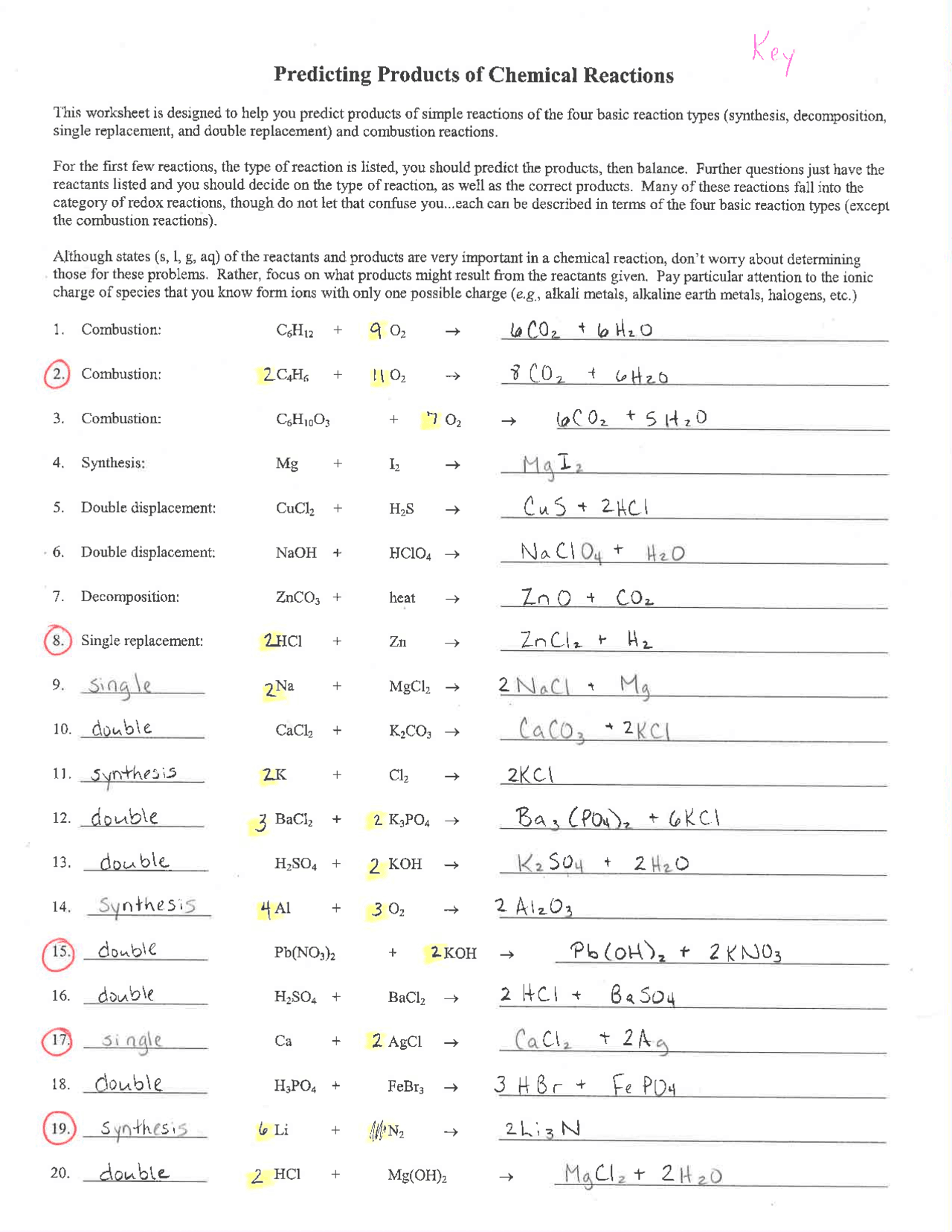
This worksheet is designed to help you predict products of simple reactions of the four basic reaction types synthesis decomposition single replacement and double replacement and combustion reactions For the first few reactions the type of reaction is listed you should predict the products then balance Nonmetal oxides g metal oxides s salts s water l acid aq individual elements look at periodic chart for state if one reactant is water then the product could be aqueous aq THE DIATOMICS H2 N2 O2 F2 Cl2 Br2 I2 DIRECTIONS Determine the products of each reaction and balance the equation Indicate all states
Decomposition And Synthesis Reactions Worksheet

Decomposition And Synthesis Reactions Worksheet
https://i.pinimg.com/originals/1e/6d/c6/1e6dc60bcc138bad961e9f4bc94b48a4.jpg
16 Types Chemical Reactions Worksheets Answers Free PDF At Worksheeto
https://www.worksheeto.com/postpic/2012/11/classifying-chemical-reactions-worksheet_243742.png
Worksheet Key For Predicting Products Of Chemical Reactions Exercises
https://static.docsity.com/documents_first_pages/2021/04/20/f08c3ce37fa53cfc6e6c2a366d4d0944.png?v=1663131013
In synthesis reactions two or more reactants come together to form one compound B AB Complete the following word equations and write and balance the formula equation calcium oxygen copper sulfur copper II sulfide 3 calcium oxide water calcium hydroxide Synthesis and Decomposition Reactions 1 For each of the following word equations write a balanced chemical equation a magnesium oxygen gas magnesium oxide 2Mg O2 2MgO b hydrogen gas oxygen gas water 2H2 O2 2H2O c potassium chlorine gas potassium chloride 2K Cl2 2KCl d iron oxygen gas iron III oxide 2Fe 3O2 2Fe2O3
A decomposition reaction is the opposite of a synthesis reaction In a decomposition reaction a single compound breaks apart into two or more elements or compounds Usually energy is required to 1 Consider the following reaction ABC energy AB BC Which type of reaction is this A decomposition reaction A synthesis reaction A reaction combining ionic bonds A
More picture related to Decomposition And Synthesis Reactions Worksheet

Synthesis And Decomposition Worksheet Addle Kindergarten
https://i.pinimg.com/736x/f8/47/4d/f8474df2157eb61f42a06c35bae26051--science-worksheets-chemical-reactions.jpg

Worksheet 1 Synthesis Decomposition Reactions Db excel
https://db-excel.com/wp-content/uploads/2019/09/worksheet-1-synthesis-decomposition-reactions.png

Decomposition And Synthesis Reactions Worksheets
https://i2.wp.com/db-excel.com/wp-content/uploads/2019/09/synthesis-and-decomposition-reactions-2.png
Name Date Types of Chemical Reactions Synthesis and Decomposition For each of the chemical reactions are listed below complete the following 0 The type ofchemical reaction synthesis or decomposition J Balance the skeletal equation I Joseph Priestley discovered oxygen gas by chemically breaking down mercury II oxide CHEMICAL REACTIONS SYNTHESIS AND DECOMPOSITION CHEMICAL REACTIONS SYNTHESIS AND DECOMPOSITION Jisela Gonzalez Member for 3 years 9 months Age 13 18 Level form 3 Language English en ID 179065 11 05 2020 Country code TT Country Trinidad Tobago School subject Chemistry 1061818
Synthesis reactions are also called combination reactions It is a reaction in which two or more substances combine to form a complex substance Synthesis reactions are generally represented as A B AB or A B C The formation of nitrogen dioxide is a synthesis reaction 2 NO g O 2 g 2 NO 2 g Worksheet 1 Synthesis Decomposition Combustion Synthesis Elements combine to form one compound OR compounds combine to form one large compound Decomposition One compound breaks apart to form elements or smaller compounds Combustion A compound burns in a reaction with oxygen

Synthesis And Decomposition Worksheet Classification Of Chemical
https://i0.wp.com/slideplayer.com/15280280/92/images/slide_1.jpg

Synthesis And Decomposition Reactions Worksheet
https://1.bp.blogspot.com/-N-T62L_XNAI/UwT06uKtznI/AAAAAAAAGFA/NOudXGAYnvc/s1600/9.++Synthesis+and+decomposition_5.jpeg
Decomposition And Synthesis Reactions Worksheet - Worksheet 1 Synthesis Decomposition Reactions Predict and balance the following synthesis and decomposition reactions Use abbreviations to indicate the phase of reactants and products where possible i e aq s l g 1 A sample of calcium carbonate is heated 2 Sulfur dioxide gas is bubbled through water 3