Complete The Balanced Neutralization Equation For The Reaction Below Example 7 4 6 7 4 6 Predicting the outcome of a neutralization reaction Write the balanced chemical equation for the neutralization of HCl with Mg OH 2 Hint neutralization reactions are a specialized type of double replacement reaction 2HCl Mg OH 2 Mg Cl 2 2H 2 O Since HCl is a strong acid and Mg OH 2 is a strong base
The products of the neutralization reaction will be water and calcium oxalate H 2 C 2 O 4 s Ca OH 2 s 2H 2 O CaC 2 O 4 s Because nothing is dissolved there are no substances to separate into ions so the net ionic equation is the equation of the three solids and one liquid Exercise 5 6 2 5 6 2 Use the calculator below to balance chemical equations and determine the type of reaction instructions Balance Chemical Equation Instructions To balance a chemical equation enter an equation of a chemical reaction and press the Balance button The balanced equation will appear above
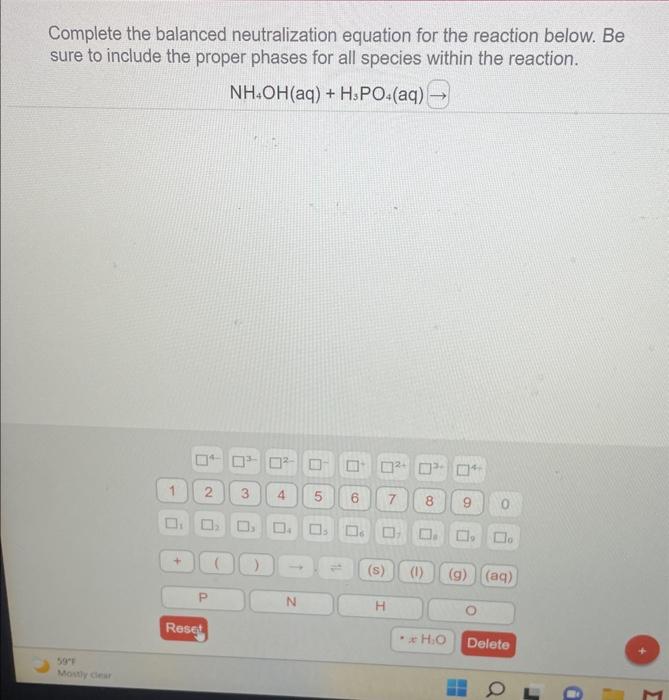
Complete The Balanced Neutralization Equation For The Reaction Below
Complete The Balanced Neutralization Equation For The Reaction Below
https://media.cheggcdn.com/media/e10/e101fc85-f8c9-4ead-ae44-c1a9878c895c/phpLNM4fp
Solved Complete The Balanced Neutralization Equation For The Chegg
https://media.cheggcdn.com/study/860/8604b5ea-470b-4a78-88c4-53ba3a510fa1/image
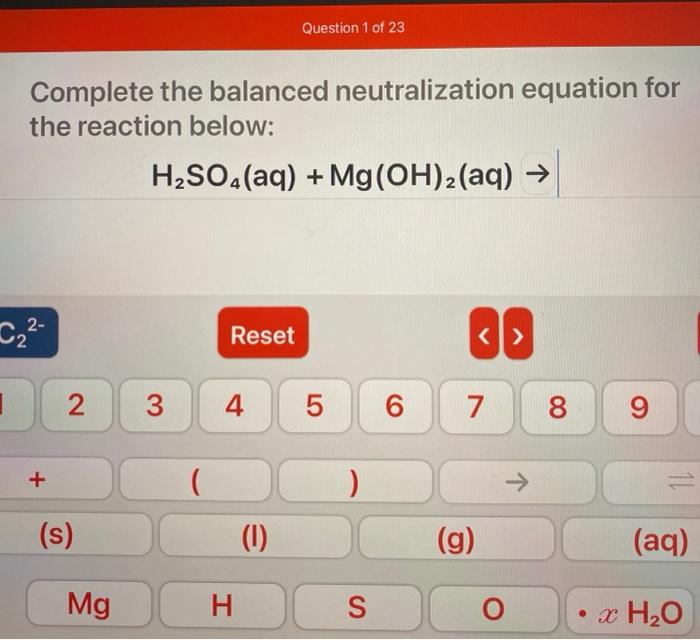
Question 1 Of 23 Complete The Balanced Neutralization Chegg
https://media.cheggcdn.com/study/571/57187581-c01f-4f21-b908-229a4731b7f3/image
Question Complete the balanced neutralization equation for the reaction below HCIO aq CsOH aq 3 L O of 1 point earned 3 attempts remaining Complete the balanced neutralization equation for the reaction below 4 H2SO4 aq Sr OH 2 aq O of 1 point earned 3 attempts remaining Complete the balanced neutralization equation for One of the most common and widely used ways to complete a neutralization reaction is through titration In a titration an acid or a base is in a flask or a beaker We will show two examples of a titration When we plug in the values given to us into the problem we get an equation that looks like the following 0 0835 0 015 M 2 0
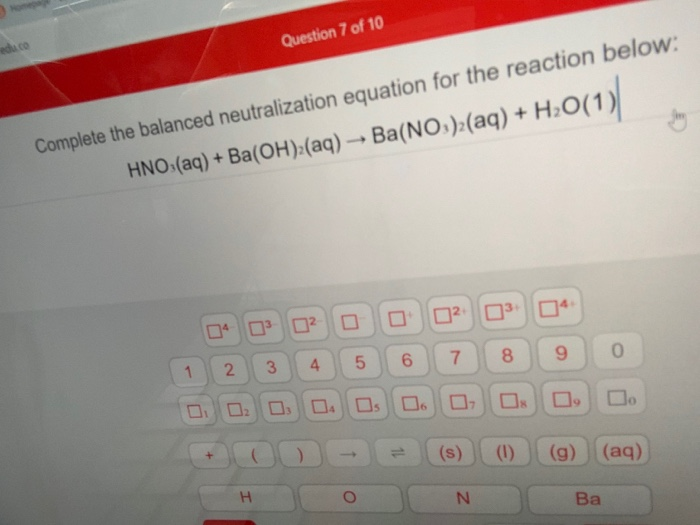
Chemistry Chemistry questions and answers Complete the balanced neutralization equation for the reaction below HNO aq Ba OH aq This problem has been solved You ll get a detailed solution from a subject matter expert that helps you learn core concepts See Answer Complete the balanced neutralization equation for the reaction below HCl aq NaOH aq Click the card to flip 1 27 Flashcards Learn Test Match Q Chat sofialasso16 Teacher Top creator on Quizlet Students also viewed English Study Words 31 terms mialufty Preview Descubre 2 Ch 5 La Ciudad vocab 14 terms ricky copelandrc Preview
More picture related to Complete The Balanced Neutralization Equation For The Reaction Below
Solved Complete The Balanced Neutralization Equation For The Reaction
https://www.coursehero.com/qa/attachment/39855198/
Solved Write The Balanced Equation For The Neutralization Reaction
https://www.coursehero.com/qa/attachment/21212698/
Solved Complete The Balance Neutralization Equation For The Reaction
https://www.coursehero.com/qa/attachment/32703641/
After that the answer is Remember that the acid molecules must donate as many hydrogen ions as many hydroxide ions you have in the base to get the same number of water molecules For example if you must neutralize aluminium hydroxide Al OH 3 with sulphuric acid H 2SO4 so you have three hydroxide ions per base formula and two hydrogen ions Home Campus Bookshelves Brevard College CHE 184 Principles of Chemistry II 7 Acid and Base Equilibria 7 17 Acids Bases Reactions Neutralization
That is ascetic acid which is reacting with strong shim hydro csic here we need to complete the neutralization reaction So as it took acid a quest but isn t reacting with strong Complete the balanced neutralization equation for the reaction below HC2H3O2 aq Sr OH 2 aq Complete and balance the equation for each of the following Complete the balanced neutralization equation for the reaction below H Cl O aq Na O H aq This problem has been solved You ll get a detailed solution from a subject matter expert that helps you learn core concepts See Answer Question Complete the balanced neutralization equation for the reaction below H Cl O aq Na O H aq
Solved Complete The Balanced Neutralization Equation not Chegg
https://media.cheggcdn.com/study/b55/b55edf98-096e-47b8-97a1-e61cea3e0480/image.png

Neutralization Reaction Definition Equation And Examples Teachoo
https://d1avenlh0i1xmr.cloudfront.net/53616fa5-7c42-4051-af1d-cef40c8ebed6/neutralisation-reaction---with-example---teachoo.jpg
Complete The Balanced Neutralization Equation For The Reaction Below - What are the reactants in the following equation HCl aq NaHCO aq CO g H O l NaCl aq A HCl NaHCO B HCl CO C NaHCO H O D CO H O E H O NaCl CO Click the card to flip A HCl NaHCO Click the card to flip 1 193 Flashcards Learn Test Match Q Chat Created by Madeline Vogl Students also viewed chem 121 exam 2