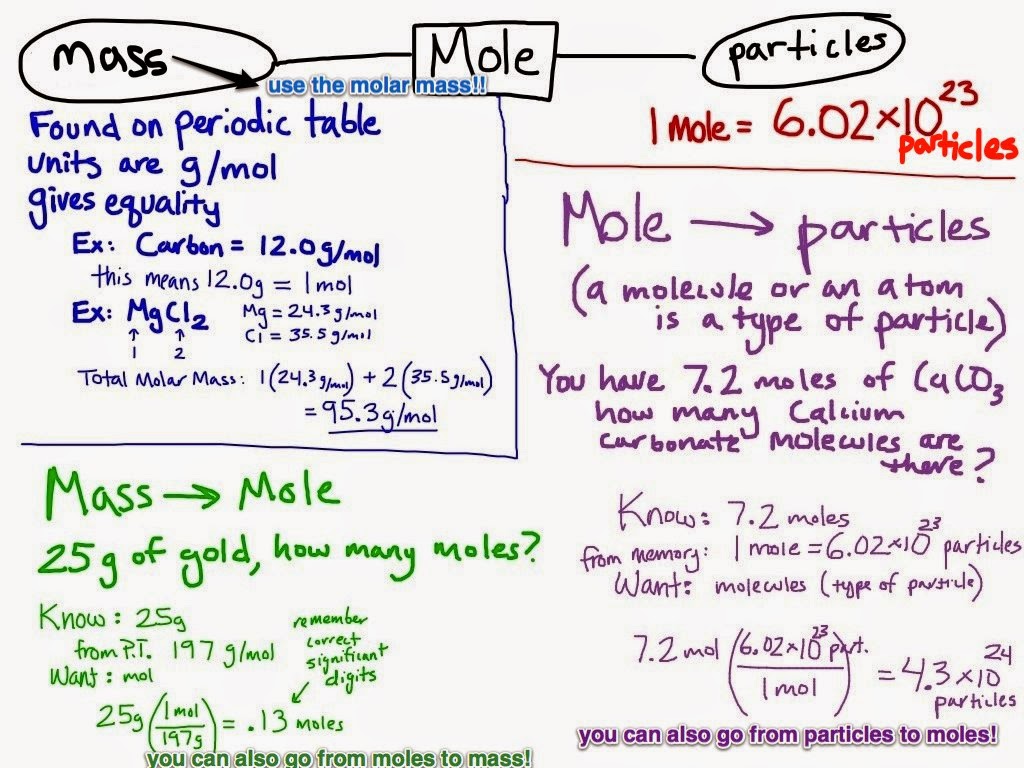
Chemistry Moles Packet The MOLE mol Unit that measures the amount number of particles of a substance 1 mol 6 02 x 1023 particles things 1 mol gram formula mass Why we use it atoms are sooooooo tiny that we have LOTS of them in a given sample
The mole is the basis of quantitative chemistry It provides chemists with a way to convert easily between the mass of a substance and the number of individual atoms molecules or formula units of that substance Conversely it enables chemists to calculate the mass of a substance needed to obtain a desired number of atoms molecules or Moles Packet INTRODUCTION TO MOLES We are about to start on a unit of chemical calculations called stoichiometry Stoichiometry is how we calculate the relationships between the amounts of reactants and the amounts of products
Chemistry Moles Packet
Chemistry Moles Packet
https://4.bp.blogspot.com/-uDaSPlTkIFM/VRFQ32j_CQI/AAAAAAAAAkE/oFRfSnmbzEo/s1600/Moles.jpg

Mole Ratio Definition And Examples
https://sciencenotes.org/wp-content/uploads/2021/01/Mole-Ratio.jpg
The Mole In Chemistry
https://s2.studylib.net/store/data/009824210_1-d5b78c5cdd9e47eed752c42f6d09ea28-768x994.png
Given the following equation complete the questions below 8Al 3Fe3O4 4Al2O3 9Fe 8 A l 3 F e 3 O 4 4 A l 2 O 3 9 F e determine the number of moles of Fe F e produced from 2 0 moles of Al A l determine the number of mol of Fe F e produced from 1 0 moles of Fe3O4 F e 3 O 4 determine the number of moles of Al2O3 A l 2 O 3 We have learned that a mole can be a certain mass of a substance and a certain number of particles A mole can also be a measure of volume when we are talking about gases You may remember from previous science classes that all gases behave basically the same as far as the physical properties of temperature pressure and volume
Lesson 2 Calculating Moles Show your work 1 Calculate the mass of 5 00 moles of water 2 Calculate the mass of 2 34 moles of Gold 3 Calculate the mass of 0 0876 moles of sodium fluoride NaF 4 Find the number of moles in 356 grams of Neon Moles and molar mass Moles and molar mass Google Classroom You might need Calculator Using the information in the table calculate the number of moles in a 2 03 kg sample of citric acid C A 6 H A 8 O A 7 Write your answer using three significant figures mol C A 6 H A 8 O A 7 Show Calculator Stuck
More picture related to Chemistry Moles Packet
AQA GCSE Chemistry Science Calculating Moles Lesson Teaching Resources
https://d1e4pidl3fu268.cloudfront.net/84dfd0c3-7207-4f4e-a577-5f6d60ffbf5e/molescover.jpg
Accelerated Chemistry Mole Review Problems Packet
http://2.bp.blogspot.com/_8T863FjT4Zo/S3DQM6OQusI/AAAAAAAAAQk/_nO9Q0pZtRI/w1200-h630-p-k-no-nu/mole+1
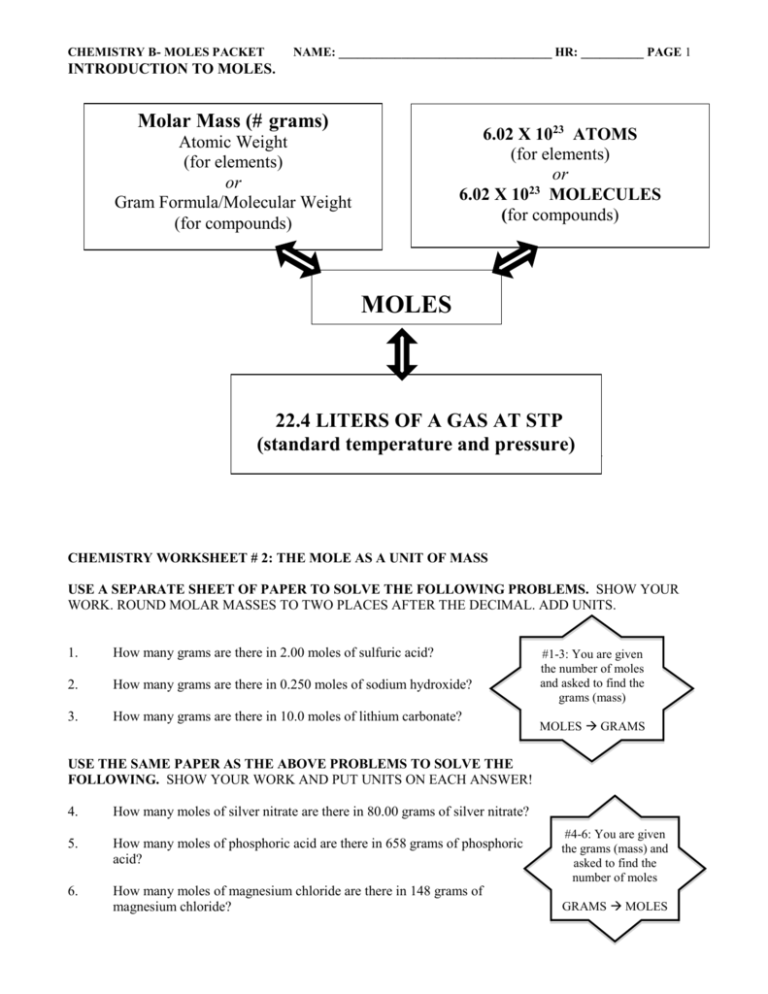
Chemistry Worksheet 2 The Mole As A Unit Of Mass
https://s3.studylib.net/store/data/007505942_1-d02a743107179b1201617bd69e496eee-768x994.png
Semester 2 This semester begins with the introduction of the mole This important concept will be used during the remainder of the year as the basis for many calculations involving chemical reactions solutions and gases In the units on thermochemistry and chemical kinetics you will learn how energy is absorbed and given off during chemical Just as a dozen implies 12 things a mole abbreviated as mol represents 6 022 1023 things The number 6 022 10 23 called Avogadro s number after the 19th century chemist Amedeo Avogadro is the number we use in chemistry to represent macroscopic amounts of atoms and molecules Thus if we have 6 022 10 23 Na atoms we say we have
1 mole 6 022 x 1023atoms molecules or ions Demonstrate an understanding of the concept of counting by mass Calculate the molar mass of a compound Determine the number of moles or number of particles in a specified mass of a substance Practice 1 Simple Mole Conversions Directions Must show all of your work with units Moles Particles 1 If you have 0 00812 mol of H 2 CO 3 how many molecules do you have 2 How many molecules of hydrofluoric acid do you have if you have 2 3 moles of hydrofluoric acid 3 How many moles of nitride ions are in 7 5 moles of Mg 3 N 2 4
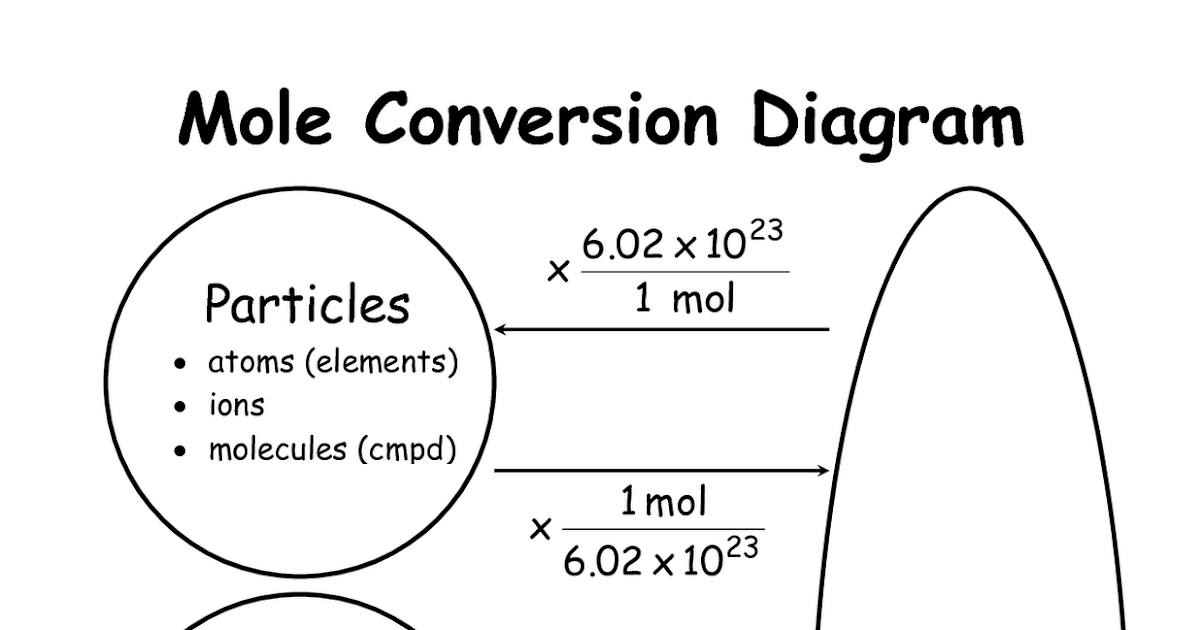
Chemistry Mysteries Mole Conversions
http://2.bp.blogspot.com/-9pWvVHv8bLM/TscWi7-jw1I/AAAAAAAAAIQ/cpJ0jZvKLks/w1200-h630-p-k-no-nu/16826138.png
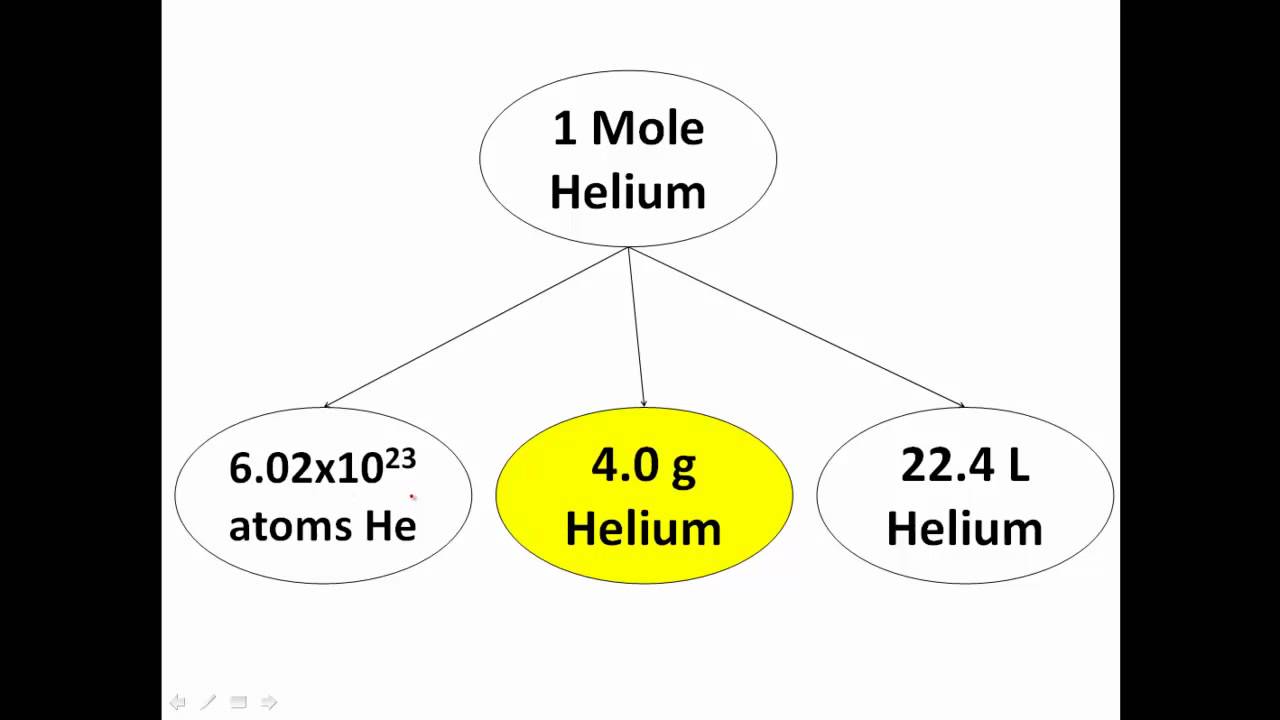
What Is A Chemistry Mole Explained YouTube
http://i1.ytimg.com/vi/JC76NR8EtTQ/maxresdefault.jpg
Chemistry Moles Packet - What is the small unit of a covalent compound An ionic compound Molar mass tells us the mass weight of 1 mol of an atom or compound In each case we simply calculate the sum of the weights of the atoms in the formula to determine the weight of a mole These weights can be found on the periodic table