Chemistry Mole Template Goosechase Flock Recommended for you Looking for a fun way for students to master mole calculations Use this template for an interactive engaging and exciting way to teach chemistry
Template for Stuffed Moles Mole Day Projects Principles of Chemistry Cut out two pieces like this for the body sides And one piece like this for the bottom Cut out four for the paws Extra details to jazz up your mole add a tail add whiskers add eyes somehow incorporate 6 02 x 1023 on your mole This video will show you step by step the process of making a mole for your chemistry class Viewers feel free to post a picture of your mole A fabulous patt
Chemistry Mole Template
/what-is-a-mole-and-why-are-moles-used-602108-FINAL-CS-01-5b7583f6c9e77c00251d4d68.png)
Chemistry Mole Template
https://www.thoughtco.com/thmb/wD11wQzyRJYPYDvUVQyrdsV0RQk=/6250x4167/filters:fill(auto,1)/what-is-a-mole-and-why-are-moles-used-602108-FINAL-CS-01-5b7583f6c9e77c00251d4d68.png

Mole Ratio Definition And Examples
https://sciencenotes.org/wp-content/uploads/2021/01/Mole-Ratio.jpg

Mole Day Projects And Ideas
https://s-media-cache-ak0.pinimg.com/originals/e8/a2/f7/e8a2f78ad3e289ec7f9203b6e603de0e.jpg
The mole is a unit used to measure the number of atoms molecules or in the case of ionic compounds formula units in a given mass of a substance The mole is defined as the amount of substance that contains the number of carbon atoms in exactly 12 g of carbon 12 and consists of Avogadro s number 6 022 10 23 of atoms of 10 The Mole The mole is the unit of measurement in the International System of Units SI for amount of substance It is defined as the amount of a chemical substance that contains as many elementary entities e g atoms molecules ions electrons or photons This number is expressed by the Avogadro constant which has a value of 6
A mole abbreviation mol is like a pair which means 2 of something You can have a pair of people a pair of apples whatever A mole is 6 022 x 10 23 of something This is a convenient quantity because it converts amu atomic mass units to grams The atomic weight of carbon is on average 12 011 amu atom Product Details Let s make a mole Celebrate Mole Day and encourage creativity in your chemistry class as students sew a mole using the mole pattern and easy to follow Make A Mole instructions
More picture related to Chemistry Mole Template
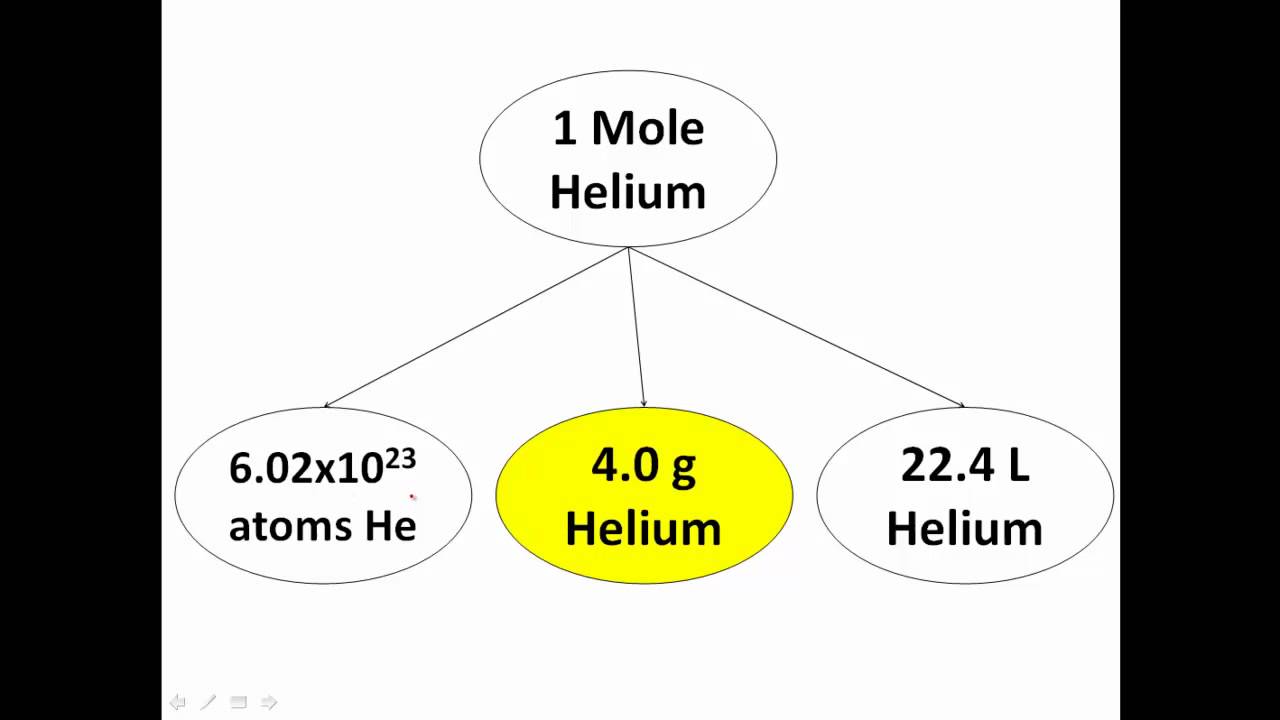
What Is A Chemistry Mole Explained YouTube
https://i.ytimg.com/vi/JC76NR8EtTQ/maxresdefault.jpg

Chemistry Mole Project Pattern Buffalohidepaintingsymbols
https://i.pinimg.com/originals/56/05/89/5605899ad9815ddcb57a00a1c20922ef.jpg

Mole Conversions And Calculation SSC Chemistry
http://sscchemistry.weebly.com/uploads/1/9/4/6/19464399/3499090_orig.jpg
A is defined as the amount of substance containing the same number of discrete entities atoms molecules ions etc as the number of atoms in a sample of pure C weighing exactly 12 g One Latin connotation for the word mole is large mass or bulk which is consistent with its use as the name for this unit Print the mole pattern Get this well in advance of the due date Using your creativity design and make your own mole The mole may be made of any fabric and any color and may have any accessories that you want to add You and another student may turn in a pair of moles if they go together in theme
Chemistry Lab Moles 1893 and it s assumed it was derived from the word Molek l molecule Ironically Ostwald s development of the mole concept was directly related to his philosophical opposition to atomic theory which he disagreed with for most of his career We can also define the mole using Avagadro s constant which is currently Types of Chemical Reactions Foldable I created this types of chemical reactions foldable for my physical science students to glue in their interactive notebooks We took a tiny break from balancing chemical equations to classify chemical equations I created this foldable to summarize the different types of reactions

SQA Higher Chemistry Mole Calculations Involving Solutions Central
https://centraltutors.co.uk/wp-content/uploads/2021/06/massMrmolestriangle.jpg
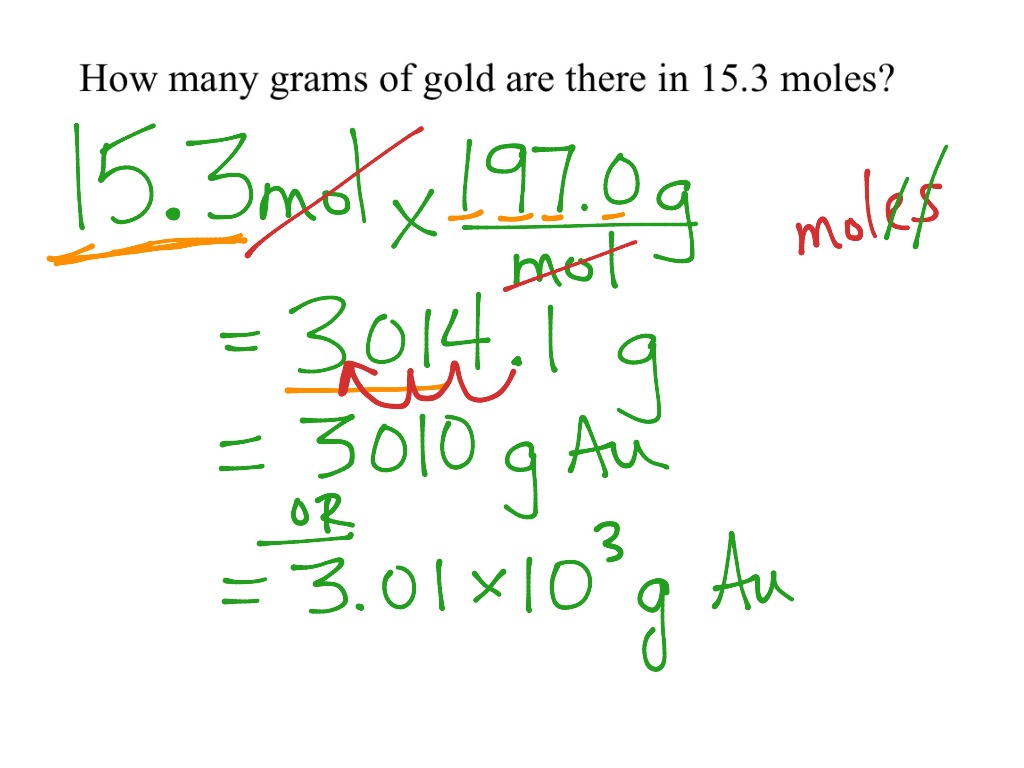
Mole Calculations CHEM 1 Science Chemistry Moles ShowMe
https://showme0-9071.kxcdn.com/files/27469/pictures/thumbs/1214078/last_thumb1383449754.jpg
Chemistry Mole Template - A mole abbreviation mol is like a pair which means 2 of something You can have a pair of people a pair of apples whatever A mole is 6 022 x 10 23 of something This is a convenient quantity because it converts amu atomic mass units to grams The atomic weight of carbon is on average 12 011 amu atom