Chemical Equilibrium Worksheet With Answers Pdf CO g 2H2 g CH3OH g an equilibrium is reached at 227 C in a 15 5 L reaction vessel with a total pressure of 6 71 102 atm It is found to contain 37 8 g of hydrogen gas 457 7 g of carbon monoxide and 7 193 g of methanol 8 Determine K and Kp where applicable for each reaction
MULTIPLE CHOICE Choose the one alternative that best completes the statement or answers the question 1 At equilibrium A the rates of the forward and reverse reactions are equal B the rate constants of the forward and reverse reactions are equal C all chemical reactions have ceased D the value of the equilibrium constant is 1 1 Equilibrium position 2 Law of chemical equilibrium 3 Reaction quotient 4 Law of mass action 5 Equilibrium constant a used to determine if a reaction has reached equilibrium b depends on the initial concentrations of the substances in a reaction c states that every reaction proceeds to an equilibrium state with a specific Keq
Chemical Equilibrium Worksheet With Answers Pdf
Chemical Equilibrium Worksheet With Answers Pdf
https://imgv2-1-f.scribdassets.com/img/document/19142211/original/4d32035aed/1614375546?v=1

SOLUTION Chem 111 Chemical Equilibrium Worksheet Answer Keys Ua
https://sp-uploads.s3.amazonaws.com/uploads/services/2005157/20210805112725_610bcb1d2be4a_chem_111_chemical_equilibrium_worksheet_answer_keys_uapage0.png

Chemical Equilibrium Worksheet Answers Free Worksheets Library Free
https://www.housview.com/wp-content/uploads/2018/01/chemical_equilibrium_worksheet_answers_free_worksheets_library_6.jpg
MODEL 1 The ICE Table A worked example Initially 1 50 moles of N2 g and 3 50 molesofH2 g were added to a 1 L container at 700 C As a result of the reaction the equilibrium concentration of NH3 g became 0 540 M QUESTION 1 1 1 A mixture of 2 mol CO g and 2 mol H2 g is sealed in a container Equilibrium with metanol CH3OH is established according to the following equation CO g 2H2 g CH3OH g Which ONE of the following statements is CORRECT At equilibrium the mixture will contain A 3 mol CH3OH B 2 mol CH3OH C 1 mol CH3OH
Characteristics used to determine if a reaction has reached equilibrium depends on the initial concentrations of the substances in a reaction states that every reaction proceeds to an equilibrium state with a specific K e q expresses the relative concentration of reactants and products at equilibrium in terms of an equilibrium constant 1 Which of the following is true for a chemical reaction at equilibrium a only the forward reaction stops b only the reverse reaction stops c both the forward and reverse reactions stop d the rate constants for the forward and reverse reactions are equal e the rates of the forward and reverse reactions are equal 2
More picture related to Chemical Equilibrium Worksheet With Answers Pdf

Chemical Equilibrium Worksheet 1 Answers Solubilidad Equilibrio Quimico
https://s3.studylib.net/store/data/007862476_2-0c02f365ec0319c27bef4f3dec52f353.png

Check Michelle Chemical Equilibrium Worksheet Answers Worksheets Nursery
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/446d2db637c76701cbc11835626d80da/thumb_1200_1554.png
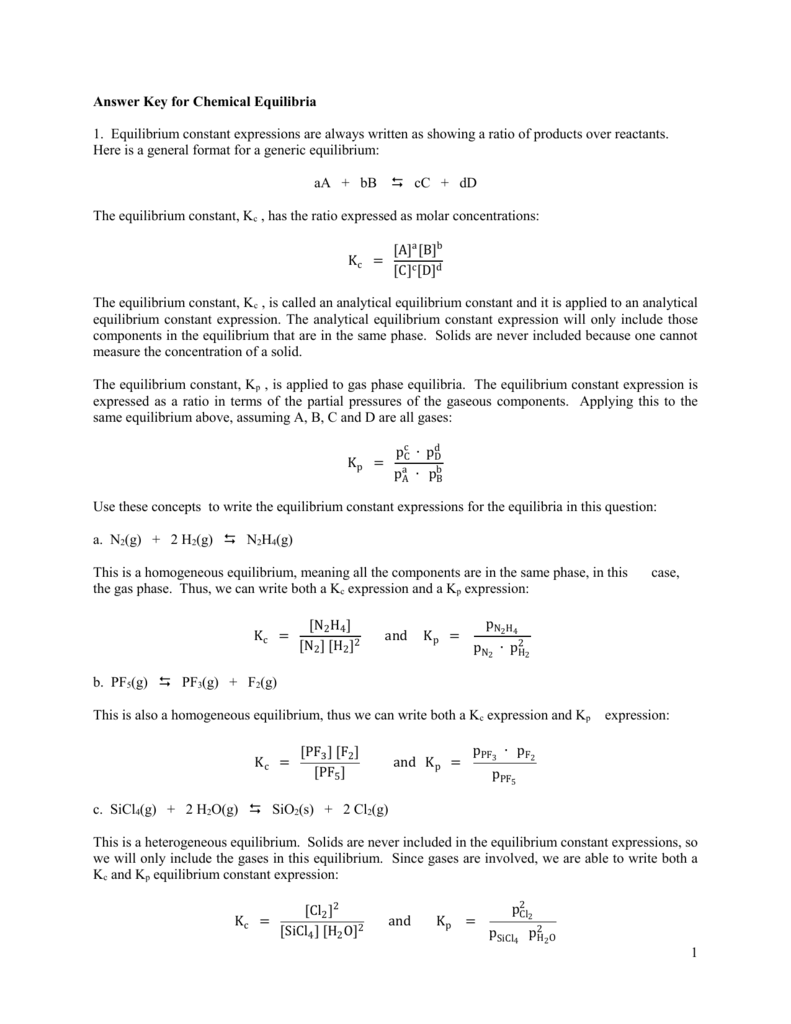
Answer Key For Chemical Equilibria 1 Equilibrium Constant
https://s3.studylib.net/store/data/006834448_2-94cf5db6d9768736bbdc397ee3d32911.png
The general form of the equilibrium constant equation is then Keq C c D d A a B b Problems involving chemical equilibria can be placed into a matrix format with two kinds of concentrations identified the initial or non equilibrium concentration Cx and the equilibrium concentration X Note the bracket versus the capital C It is the Section 17 1 Equilibrium State and Equilibrium Constant Chemical reactions do NOT go to completion 100 products even those that look like they do Reactions instead reach a point called equilibrium after which the amount of reactants and products no longer change with time This is because all reactions are
Chemical equilibrium occurs when a reaction and its reverse reaction proceed at the same rate The Concept of Equilibrium As a system approaches equilibrium both the forward and reverse reactions are occurring At equilibrium the forward and reverse reactions are proceeding at the same rate A System at Equilibrium Equilibrium Worksheet 2 Chemists believe that chemical reactions occur because the molecules involved in the reaction A exist only below a certain maximum temperature B are always unstable C are moving so fast that the chance of interaction is very small D spontaneously break apart then recombine E collide with each

Chemical Equilibrium Worksheet With Answers Pdf Exam Academy
https://tomdunnacademy.org/pictures/picture_3126.jpg

Chemical Equilibrium Worksheet 1 Answers Solubilidad Equilibrio Quimico
https://www.housview.com/wp-content/uploads/2018/07/chemical_equilibrium_worksheet_answers_4.jpg
Chemical Equilibrium Worksheet With Answers Pdf - MODEL 1 The ICE Table A worked example Initially 1 50 moles of N2 g and 3 50 molesofH2 g were added to a 1 L container at 700 C As a result of the reaction the equilibrium concentration of NH3 g became 0 540 M
