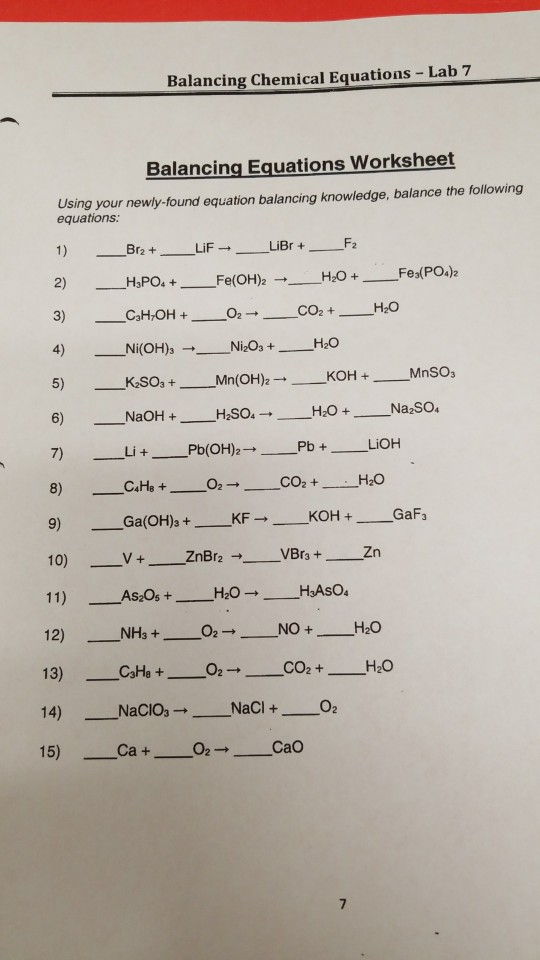
Chapter 7 Worksheet 1 Balancing Chemical Equations Chapter 7 Worksheet 1 Balancing Chemical Equations Zinc and lead II nitrate react to form zinc nitrate and lead Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas Sodium phosphate and calcium chloride react to form calcium phosphate and sodium chloride Potassium metal and chlorine gas combine to form
The law of conservation of mass does not apply to skeletal equations The chemical formulas are represented by balanced chemical equations which follow the law of conservation of mass which states that the atoms on the reactant and product sides are the same H 2 O H 2 O 2 Skeletal equation 2H 2 O 2H 2 O 2 Balanced chemical Balancing Chemical Equations Answer Key Balance the equations below N2 3 H2 2 NH3 KClO3 2 KCl 3 O2 2 NaCl 1 F2 2 NaF 1 Cl2 2 H2 1 O2 2 H2O Pb OH 2 2 HCl 2 H2O 1 PbCl2 AlBr3 3 K2SO4 6 KBr 1 Al2 SO4 3 CH4 2 O2 1 CO2 2 H2O
Chapter 7 Worksheet 1 Balancing Chemical Equations

Chapter 7 Worksheet 1 Balancing Chemical Equations
https://db-excel.com/wp-content/uploads/2019/09/balancing-chemical-equations-docsity-750x970.png

Download Balancing Equations 26 Balancing Equations Equations
https://i.pinimg.com/originals/e0/b9/7b/e0b97b801a43dc168395b1d492c27010.jpg
49 Balancing Chemical Equations Worksheets with Answers Worksheet
https://lh5.googleusercontent.com/proxy/R_rfkBkGT8IFYDIsuni9wzwCAubKpe9sTDAGTh01rRv_RvM5xyJj6z9OZd7rt3pUOLFoSntZ2FkGfBEj1wlYCI2ajYDWJ_KoESYu0lRVS3vj5fbEZBS5xsao3SYrFLGfuHncQaRGD0XbGG6xzUfm6ccDs6a9XbE77N6seA=s0-d
The chemical equation described in section 4 1 is balanced meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter It may be confirmed by simply summing the numbers of 4 1 4 2 2 4 4 4 yes O 2 2 4 1 2 2 1 4 4 4 yes A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection Consider as an example the decomposition of water to yield molecular hydrogen and oxygen
C S O2 SO 3 d 2S 3O2 2SO 3 d 2S 3O2 2SO 3 Because the question was basically asking to balance for oxygen to react to sulfur trioxide When magnesium carbonate MgCO2 reacts with nitric acid HNO3 magnesium nitrate and carbonic acid form Carbonic acid then breaks down into water and carbon dioxide Balance the following chemical equation Mg OH 2 HCl MgCl 2 H 2 O Note All reactants and products require a coefficient of at least one Stuck Review related articles videos or use a hint
More picture related to Chapter 7 Worksheet 1 Balancing Chemical Equations

Balancing Equations Answer Key Balancing Math Equations Balancing
https://images-na.ssl-images-amazon.com/images/I/81ixpcdJRVL.jpg
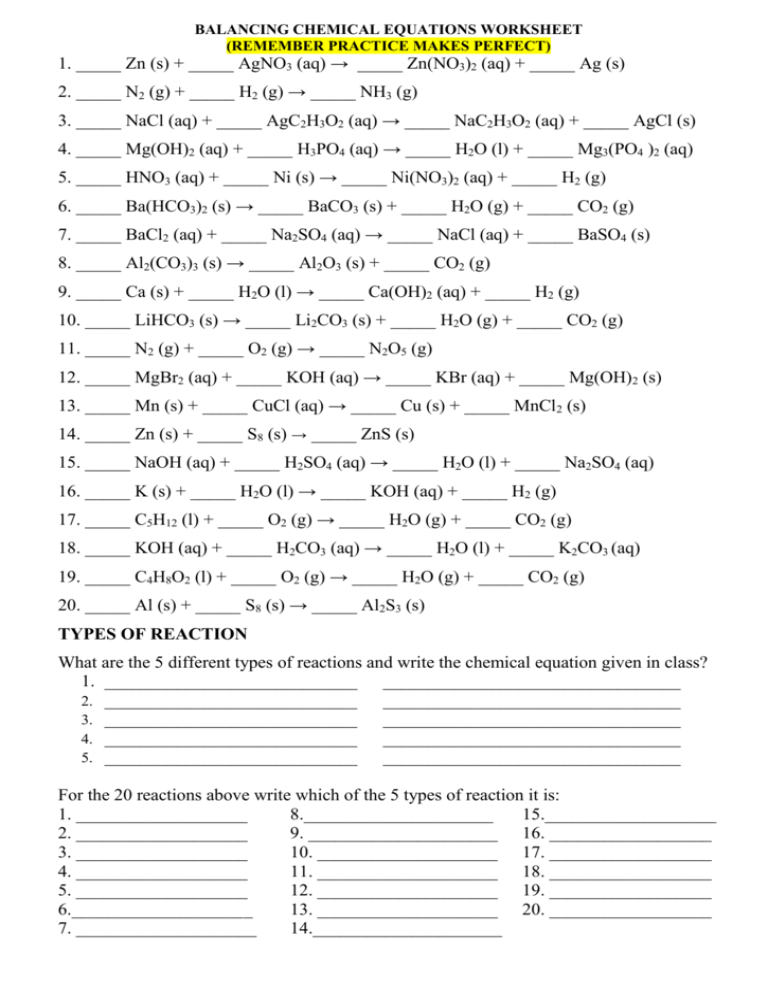
BALANCING CHEMICAL EQUATIONS WORKSHEET
https://s3.studylib.net/store/data/008944509_1-3eaef57724735f2a4413388c3e79de75-768x994.png
Answer Key Balancing Equations Worksheet Displaying 8 Worksheets For
https://d2vlcm61l7u1fs.cloudfront.net/media/ece/ece8d0e0-7d2e-41d8-9d13-4c1b348fa230/image
Balancing chemical equations with key pdf Free download as PDF File pdf Text File txt or read online for free Scribd is the world s largest social reading and publishing site C 2H 6 7 2O 2 3H 2O 2CO 2 A conventional balanced equation with integer only coefficients is derived by multiplying each coefficient by 2 2C 2H 6 7O 2 6H 2O 4CO 2 Finally with regard to balanced equations recall that convention dictates use of the smallest whole number coefficients
Download Lecture notes Chapter 7 Worksheet 1 Balancing Chemical Equations Universiteit Antwerpen UFSIA Chapter 7 Worksheet 1 Balancing Chemical Equations Balance the equations below 1 Balancing Chemical Equations Answer Key Step 1 Write Down the Unbalanced Equation The first step to balance the equation is to write down the chemical formula of reactants that are listed on the left side of the chemical equation After this you can list down the products on the right hand side of the chemical equation

Chapter 7 Worksheet 1 Balancing Chemical Equations Printable Word
https://i2.wp.com/westernmotodrags.com/wp-content/uploads/2018/08/balancing-chemical-equations-worksheets-with-answers-balancing-chemical-equations-worksheet-google-search-science-balance.jpg
Solved Balance The Equations Below 1 N 2 H 2 Chegg
http://media.cheggcdn.com/media/860/860ea149-8563-4052-afaf-b579eebd6888/image
Chapter 7 Worksheet 1 Balancing Chemical Equations - View Chapter 7 Chem 101 worksheet Balancing Chemical Equations docx from SO 4 at Lake Michigan College Chem 101 Chapter 7 Worksheet 1 Balancing Chemical Equations Name Alejandro Jimenez Balance