Chapter 7 Review Chemical Formulas And Chemical Compounds Chapter 7 Chemical Formulas and Chemical Compounds Review Flashcards Quizlet Chapter 7 Chemical Formulas and Chemical Compounds Review The oxidation number of fluorine is Click the card to flip B 1 in all compounds Click the card to flip 1 42 Flashcards Learn Test Match Q Chat Created by Heather5600 Students also viewed
Start studying Chapter 7 review chemical formulas and chemical compounds Learn vocabulary terms and more with flashcards games and other study tools Chapter 7 Chemical Formulas and Chemical Compounds What follows an element s symbol in a chemical formula to indicate the number of atoms that element in one molecule of the compound a charge sign b super scripted number c number in parenthesis d sub scripted number Click the card to flip d Click the card to flip 1 26 Flashcards Learn
Chapter 7 Review Chemical Formulas And Chemical Compounds

Chapter 7 Review Chemical Formulas And Chemical Compounds
https://image2.slideserve.com/4565038/chapter-7-chemical-formulas-chemical-compounds-l.jpg
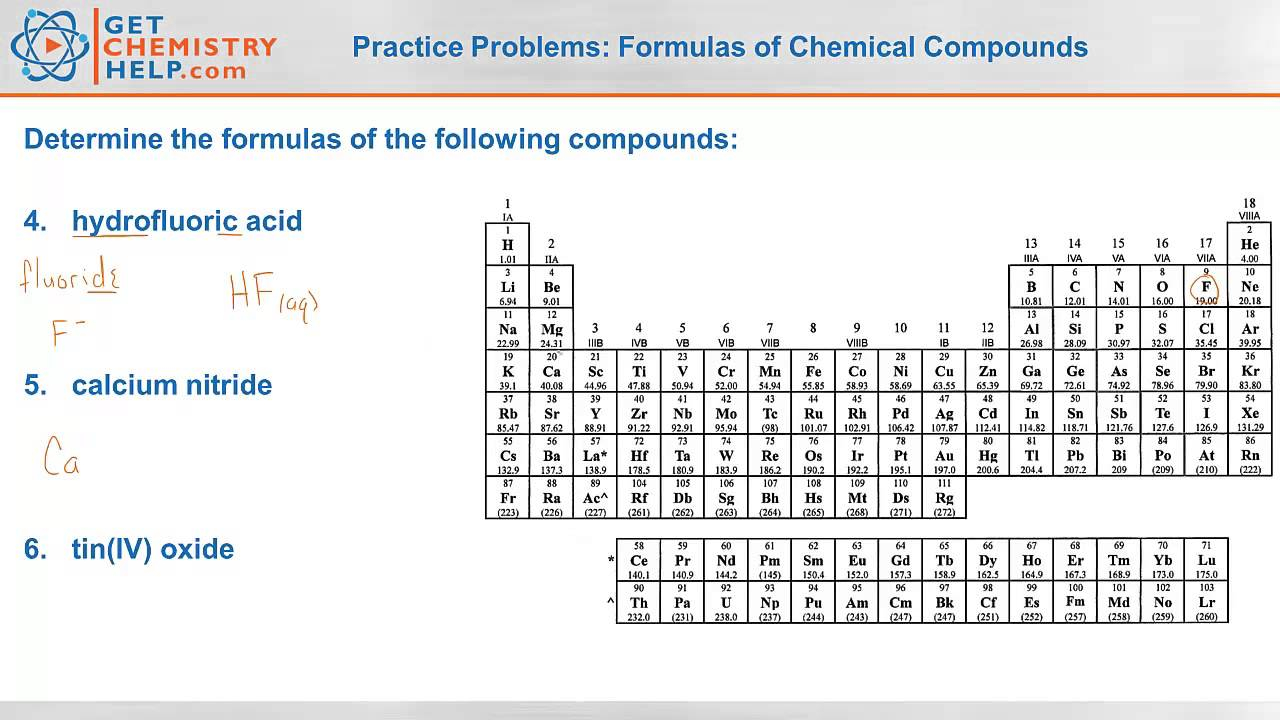
Chemical Formulas And Chemical Compounds Chapter 7 Chemical Formulas
https://www.compoundworksheets.com/wp-content/uploads/2023/03/chemical-formulas-and-chemical-compounds-chapter-7-chemical-formulas.jpg

11 Chapter 7 Review Chemical Formulas And Chemical Compounds Answer
https://s3.studylib.net/store/data/008564810_1-caa29f99b4fa46cfa965a01a6abeb185.png
1 Write formulas for the following compounds a copper II carbonate b sodium sulfite c ammonium phosphate d tin IV sulfide e nitrous acid 2 Write the Stock system names for the following compounds a Mg ClO4 2 b Fe NO3 2 c Fe NO2 3 d CoO e dinitrogen pentoxide 3 a How many atoms are represented by the formula Ca HSO4 2 b A 174 g b 1 81 10 molecules c C H Naphthalene is a soft covalent solid that is often used in mothballs Its molar mass is 128 18 g mol and it contains 93 75 carbon and 6 25 hydrogen Determine the molecular formula of naphthalene from this information
a changes the number of moles represented by the formula b changes the charges on the other ions in the compound c changes the formula so that it no longer represents the compound it previously represented d has no effect on the formula 3 The explosive TNT has the molecular formula C7H5 NO2 3 For a molecular compound the chemical formula reveals the number of atoms of each element contained in a single molecule of the compound as shown below for the hydrocarbon octane Hydrocarbonsare molecular compounds composed solely of carbon and hydrogen
More picture related to Chapter 7 Review Chemical Formulas And Chemical Compounds

Chapter 7 Chemical Formulas And Chemical Compounds
https://s2.studylib.net/store/data/009830778_1-821fed695b08663b0cb29f85113aacce-768x994.png

Chapter 7 Chemical Formulas Compounds
https://s2.studylib.net/store/data/009841455_1-471c56c670c4709293dd448ae8ca4dc9-768x994.png
PPT Chapter 7 Chemical Formulas And Chemical Compounds PowerPoint
https://image2.slideserve.com/3913420/chapter-7-chemical-formulas-and-chemical-compounds-l.jpg
CHAPTER 7 REVIEW Chemical Formulas and Chemical Compounds SECTION 3 SHORT ANSWER Answer the following questions in the space provided 1 Label each of the following statements as True or False 2 How many moles of each element are present in a 10 0 mol sample of Ca NO3 2 For anions For metals that form only one ion E Compounds Containing Polyatomic Ions Oxyanions Polyatomic anions that contain oxygen Naming a series of similar polyatomic ions ClO Hypochlorite ClO2 Chlorite ClO3 Chlorate ClO4 Perchlorate
The chemical formula for an ionic compound represents one formula unit the simplest ratio of the compound s positive ions cations and its negative ions anions example aluminum sulfate Al2 SO4 3 Parentheses surround the polyatomic ion SO 2 4 to identify it as a unit The subscript 3 refers to the unit 1 minute 1 pt A chemical formula includes the symbols of the elements in the compound and subscripts that indicate atomic mass of each element number of atoms or ions of each element that are combined in the compound formula mass charges on the elements or ions 2 Multiple Choice

Chapter 7 Test Review
https://s2.studylib.net/store/data/010001682_1-a56a9c9c31062501a2ae9a36de785cbf.png
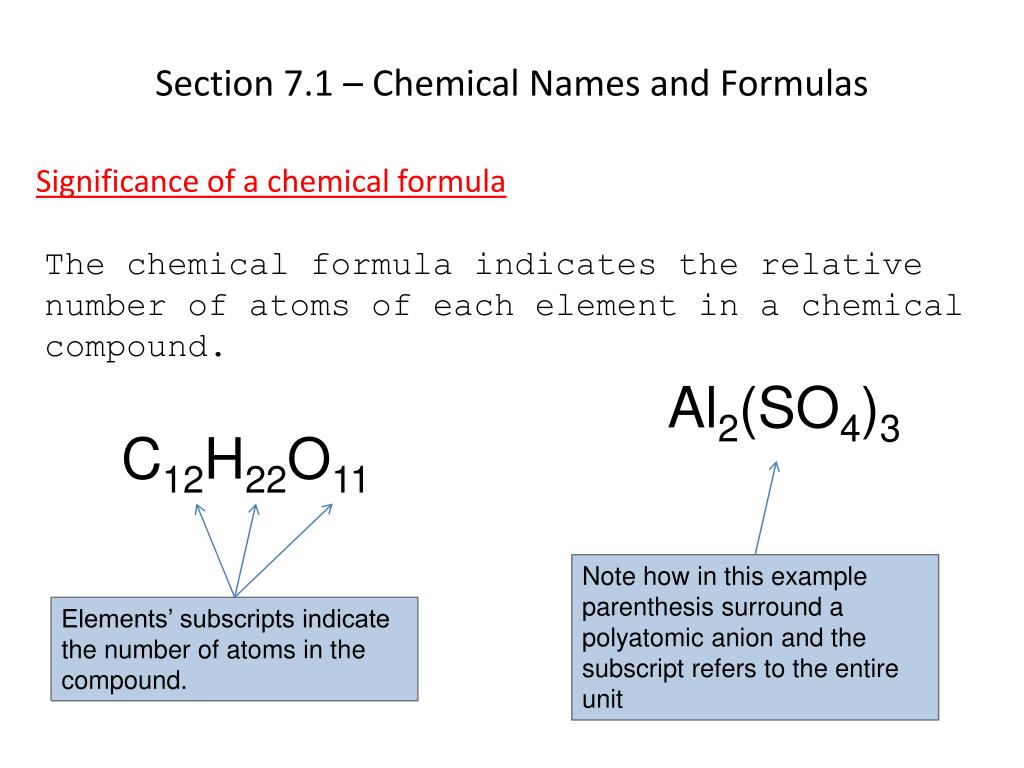
Ppt Chapter 7 Chemical Formulas And Chemical Compounds Powerpoint Riset
https://image2.slideserve.com/4887955/section-7-1-chemical-names-and-formulas1-l.jpg
Chapter 7 Review Chemical Formulas And Chemical Compounds - A 174 g b 1 81 10 molecules c C H Naphthalene is a soft covalent solid that is often used in mothballs Its molar mass is 128 18 g mol and it contains 93 75 carbon and 6 25 hydrogen Determine the molecular formula of naphthalene from this information