Calculating Average Atomic Mass Worksheet Atomic Mass Total 9 22 Average Atomic Mass Worksheet Solutions 1 Rubidium has two common isotopes 85Rb and 87Rb If the abundance of 85Rb is 72 2 and the abundance of 87Rb is 27 8 what is the average atomic mass of rubidium 85 56 amu 2 Uranium has three common isotopes
Use the atomic masses of each of the two isotopes of chlorine along with their respective percent abundances to calculate the average atomic mass of chlorine Step 1 List the known and unknown quantities and plan the problem Change each percent abundance into decimal form by dividing by 100 Calculate the average atomic mass of the element nitrogen N using the following data Isotope abundance Nitrogen 14 95 Nitrogen 15 3 Nitrogen 16 2 7 Calculate the average atomic mass of the element Iodine I using the following data Isotope abundance Iodine 127 80 Iodine 126 17 Iodine 128 3 8
Calculating Average Atomic Mass Worksheet

Calculating Average Atomic Mass Worksheet
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/190b01343490a8257c1a5d2a56d9a2be/thumb_1200_1553.png

Calculating Average Atomic Mass Worksheet Printable Worksheets And
https://i0.wp.com/www.fabtemplatez.com/wp-content/uploads/2018/03/average-atomic-mass-worksheet-answer-key-90473-molar-mass-worksheet-answers-with-work-worksheets-for-all-average-atomic-mass-worksheet-answer-key-15311197.jpg

Calculating Average Atomic Mass Worksheet
https://s3.studylib.net/store/data/008549995_1-ed2f631999dd462990e0c96ed9c082ca.png
To calculate average atomic mass of an element Average atomic mass fractional abundance of isotope 1 atomic mass of isotope 1 fractional abundance of isotope 2 atomic mass of isotope 2 Practice Problems 1 Chlorine has two isotopes Chlorine 35 has an actual mass of 34 9689 u and chlorine 37 has a mass of 36 9659 u Find the average atomic mass for Mg if 78 99 of Mg atoms are 24Mg with a mass of 23 9850419 amu 10 00 are 25Mg with a mass of 24 9858370 amu and 11 01 are 26Mg with a mass of 25 9825930 amu Calculate the average atomic mass for each element based on the natural abundance of its isotopes Learn with flashcards games and more for free
A Use the equation in question 1 to calculate the atomic mass of an element that has two isotopes each with 50 00 abundance One isotope has a mass of 63 00 amu and the other has a mass of 68 00 amu b Recalculate the atomic mass if instead there is 80 00 of the 63 00 amu isotope and 20 00 of the 68 00 amu isotope Calculate the average atomic mass of sulfur if 95 00 of all sulfur atoms have a mass of 31 972 amu 0 76 has a mass of 32 971amu and 4 22 have a mass of 33 967amu 4 Calculate the average atomic mass of bromine One isotope of bromine has an atomic mass of 78 92amu and a relative abundance of 50 69
More picture related to Calculating Average Atomic Mass Worksheet

50 Calculating Average Atomic Mass Worksheet
https://chessmuseum.org/wp-content/uploads/2019/10/calculating-average-atomic-mass-worksheet-new-atomic-mass-atomic-number-and-isotopes-of-calculating-average-atomic-mass-worksheet.png

Free Printable Calculating Average Atomic Mass Worksheets
https://storage.googleapis.com/worksheetzone/image/63ac01e5e670694cee59cf4a/calculating-average-atomic-mass-worksheet-w1000-h1294-preview-0.png

Free Printable Calculating Average Atomic Mass Worksheets
https://storage.googleapis.com/worksheetzone/image/63ac1e01e670694cee5a6992/average-atomic-mass-calculations-w1000-h1294-preview-0.png
This worksheet will show students how these numbers are calculated and help them understand why the atomic mass of oxygen is 15 99 AMU instead of simply 16 AMU Essential Concepts Isotopes average atomic mass isotope abundance atomic mass units Answer key Included in the chemistry instructor resources subscription Click here for details Calculate the average atomic mass of copper Calculate the average atomic mass of Sulfur if 95 00 of all sulfur atoms have a mass of 31 972 amu 0 76 have a mass of 32 971amu and 4 22 have a mass of 33 967amu The four isotopes of Lead are shown below each with its percent by mass abundance and the composition of its nucleus
Calculate the average atomic masses Round all answers to two decimal places 1 What is the atomic mass of hafnium if out of every 100 atoms 5 have a mass of 176 19 have a mass of 177 27 have a mass of 178 14 have a mass of 179 and 35 have a mass of 180 0 2 Iodine is 80 127I 17 126I and 3 128I Worksheets Average Atomic Mass 2 Average Atomic Mass 2 jlinde Member for 1 year 2 months Age 15 Level 10 Language English en ID 2231499 01 12 2022 Country code US Country United States School subject Chemistry 1061818 Main content Atomic Mass 2104157 Practice Calculating Average Atomic Mass Other contents Average Atomic Mass

Free Printable Calculating Average Atomic Mass Worksheets
https://storage.googleapis.com/worksheetzone/image/63ac1ed7e670694cee5a7134/calculating-average-atomic-mass-w1000-h1294-preview-1.png
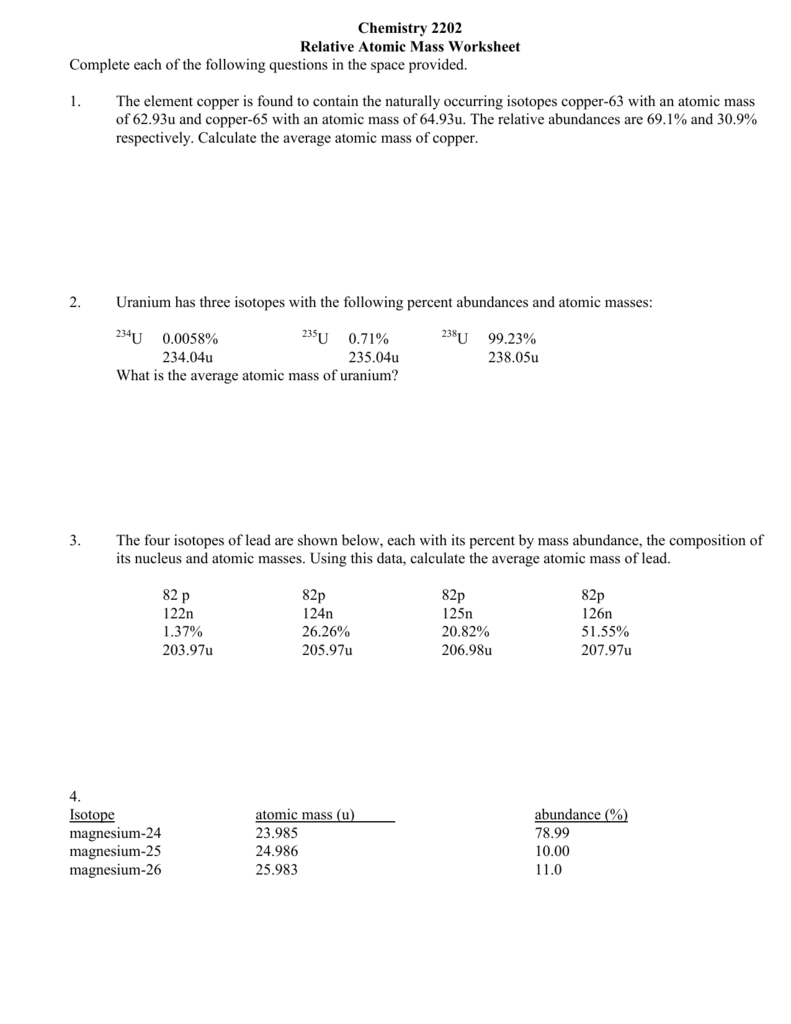
Average Atomic Mass Worksheet
https://s3.studylib.net/store/data/008342063_1-db5fd2c98d3957944061536c714386ef.png
Calculating Average Atomic Mass Worksheet - PROBLEM PageIndex 4 Average atomic masses listed by IUPAC are based on a study of experimental results Bromine has two isotopes 79 Br and 81 Br whose masses 78 9183 and 80 9163 amu and abundances 50 69 and 49 31 were determined in earlier experiments Calculate the average atomic mass of Br based on these experiments