Calculating Average Atomic Mass Worksheet Answer Key Answer The atomic mass of boron is 10 811 therefore boron 11 is more abundant because the mass number is closer to the atomic mass 8 Lithium 6 is 4 abundant and lithium 7 is 96 abundant What is the average mass of lithium Answer 6 96 amu 9 Iodine is 80 127I 17 126I and 3 128I Calculate the average atomic mass of iodine Answer
Use the atomic masses of each of the two isotopes of chlorine along with their respective percent abundances to calculate the average atomic mass of chlorine Step 1 List the known and unknown quantities and plan the problem Change each percent abundance into decimal form by dividing by 100 69 for mass of 62 30 for mass of 64 Calculate the average atomic mass of Copper Show ALL work for full credit 69 100 x 62 43 30 100 x 64 19 ggghvhvhvhvhhv 63 Calculate the average atomic mass of Sulfur if 95 of all Sulfur atoms have a mass of 31 u 0 has a mass of 32 and 4 have a mass of 33
Calculating Average Atomic Mass Worksheet Answer Key

Calculating Average Atomic Mass Worksheet Answer Key
https://s3.studylib.net/store/data/005882184_1-0530358f4674ae7f501a552b6efd82a5.png

Calculating Isotopes Worksheet Printable Word Searches
https://i0.wp.com/www.worksheeto.com/postpic/2009/12/average-atomic-mass-and-isotopes-worksheet-answer-key_212396.png?crop=12
Calculating Atomic Mass Worksheets Answers
http://www.unmisravle.com/wp-content/uploads/2018/03/average_atomic_mass_worksheet_answer_key_1.jpg
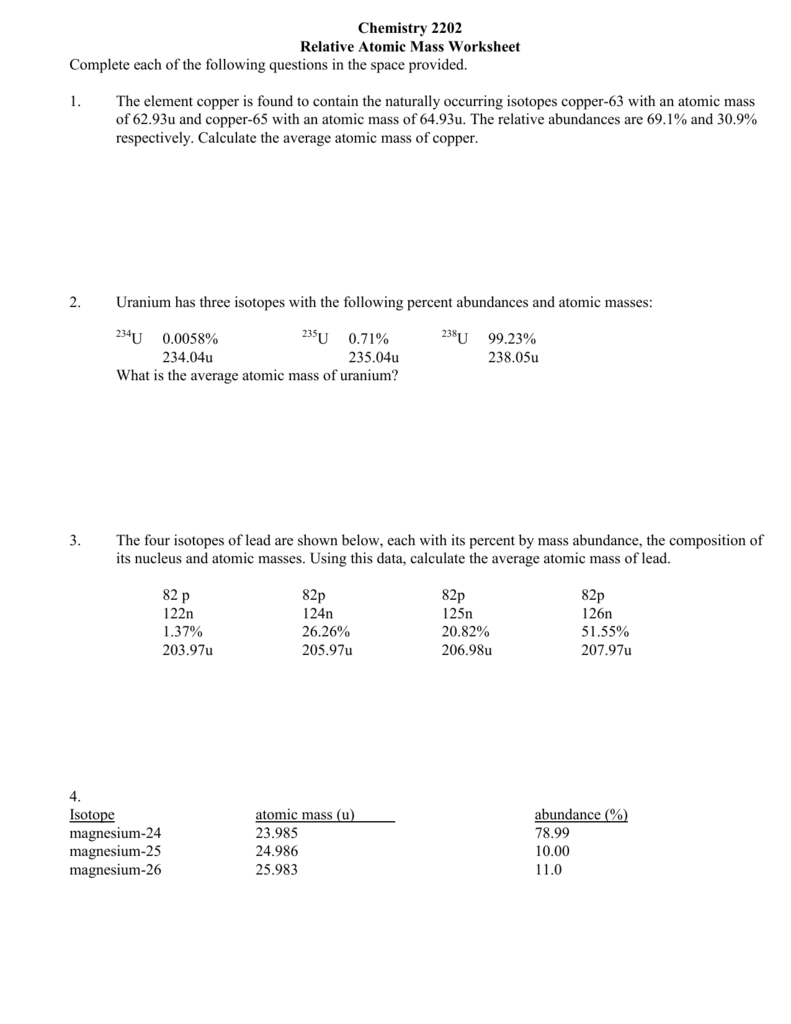
And 24 47 percent 37Cl mass 36 966 amu Calculate the average atomic mass 6 Copper used in electric wires comes in two flavors isotopes 63Cu and 65Cu 63Cu has an atomic mass of 62 9298 amu and an abundance of 69 09 The other isotope 65Cu has an abundance of 30 91 The average atomic mass between these two isotopes is 63 546 amu Find the average atomic mass for Mg if 78 99 of Mg atoms are 24Mg with a mass of 23 9850419 amu 10 00 are 25Mg with a mass of 24 9858370 amu and 11 01 are 26Mg with a mass of 25 9825930 amu Calculate the average atomic mass for each element based on the natural abundance of its isotopes Learn with flashcards games and more for free
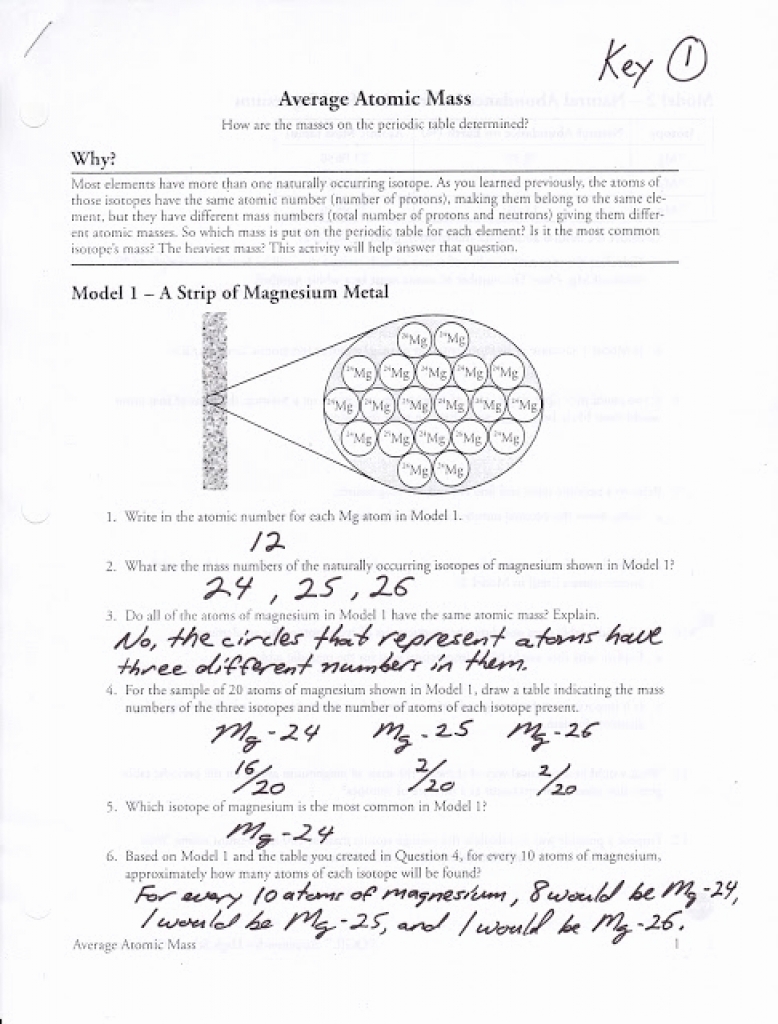
37 Calculate the average atomic mass of chlorine 2 Copper has two isotopes Copper 63 which has an atomic mass of 62 93 u and copper 65 which has an atomic mass of 64 93 u In any sample of copper atoms 69 1 will be copper 63 and 30 9 will be copper 65 Calculate the average atomic mass of naturally occurring copper 3 One atom has 20 Atomic Mass Because each proton and each neutron contribute approximately one amu to the mass of an atom and each electron contributes far less the atomic mass of a single atom is approximately equal to its mass number a whole number However the average masses of atoms of most elements are not whole numbers because most elements exist naturally as mixtures of two or more isotopes
More picture related to Calculating Average Atomic Mass Worksheet Answer Key
Average Atomic Mass Worksheet
https://s3.studylib.net/store/data/008342063_1-db5fd2c98d3957944061536c714386ef.png

Understanding The Average Atomic Mass Worksheet Free Worksheets
https://i2.wp.com/briefencounters.ca/wp-content/uploads/2018/11/average-atomic-mass-worksheet-show-all-work-answer-key-as-well-as-acids-and-bases-introduction-worksheet-answers-of-average-atomic-mass-worksheet-show-all-work-answer-key.jpg?fit=1721%2C2048&ssl=1

Calculating Average Atomic Mass Worksheet Name
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/190b01343490a8257c1a5d2a56d9a2be/thumb_1200_1553.png
ID 2231499 01 12 2022 Country code US Country United States School subject Chemistry 1061818 Main content Atomic Mass 2104157 Practice Calculating Average Atomic Mass Other contents Average Atomic Mass Loading ad Practice calculating average atomic mass with this 12 problem worksheet Perfect for classwork homework extra practice or as examples for students in a distance learning setting This product includes the following as a PDF file 12 Problems Calculating Average Atomic Mass Answer Key
Calculate the average atomic mass of Sulfur if 95 of all Sulfur atoms have a mass of 31 u 0 has a mass of 32 and 4 have a mass of 33 Show ALL work for full credit Naturally occurring Strontium consists of four isotopes Sr 84 Sr 86 Sr 87 and Sr 88 Write the atomic symbol symbol notation for the two isotopes of uranium U whose atomic number is 92 One isotope has 142 neutrons and the other isotope has 146 neutrons 5 Calculate the average atomic mass of the element iron Fe using the following data Isotope 6 Calculate the average atomic mass of the element nitrogen N using the

Calculating Average Atomic Mass Worksheet Worksheet Gambaran
https://s3.studylib.net/store/data/025337855_1-1d532e107d8c14eb1aee12907c17193b.png

Calculating Average Atomic Mass Worksheet
http://www.housview.com/wp-content/uploads/2018/05/calculating_average_atomic_mass_worksheet_answers_best_of_electron_8.jpg
Calculating Average Atomic Mass Worksheet Answer Key - 37 Calculate the average atomic mass of chlorine 2 Copper has two isotopes Copper 63 which has an atomic mass of 62 93 u and copper 65 which has an atomic mass of 64 93 u In any sample of copper atoms 69 1 will be copper 63 and 30 9 will be copper 65 Calculate the average atomic mass of naturally occurring copper 3 One atom has 20