Calculating Atomic Mass Worksheet Learn Atomic Mass with free step by step video explanations and practice problems by experienced tutors Worksheet The Atom 0 Subatomic Particles 0 Isotopes 0 Ions 0 Atomic Mass 0 207 9767 u 52 4 Calculate the atomic mass of lead 8 PRACTICE PROBLEM Below is an image showing 30 atoms of a hypothetical element Az
This worksheet will provide you with the necessary background and practice to be a pro at working on atomic mass calculations textbook section 4 9 Part A Background information for atomic mass calculations 1 Write down the equation from your textbook or lecture notes for calculating atomic mass from isotope masses and natural abundances 37 Calculate the average atomic mass of chlorine 2 Copper has two isotopes Copper 63 which has an atomic mass of 62 93 u and copper 65 which has an atomic mass of 64 93 u In any sample of copper atoms 69 1 will be copper 63 and 30 9 will be copper 65 Calculate the average atomic mass of naturally occurring copper 3 One atom has 20
Calculating Atomic Mass Worksheet
Calculating Atomic Mass Worksheet
https://www.pdffiller.com/preview/42/740/42740409/large.png
Average Atomic Mass Worksheet
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/7086b4569d1b03b77258b8edb77ce461/thumb_1200_1553.png
Calculating Average Atomic Mass Worksheet
http://www.housview.com/wp-content/uploads/2018/05/calculating_average_atomic_mass_worksheet_answers_best_of_electron_8.jpg
The mass of one atom is usually expressed in atomic mass units amu which is referred to as the atomic mass An amu is defined as exactly 1 12 1 12 of the mass of a carbon 12 atom and is equal to 1 6605 10 24 g Protons are relatively heavy particles with a charge of 1 and a mass of 1 0073 amu And 24 47 percent 37Cl mass 36 966 amu Calculate the average atomic mass 6 Copper used in electric wires comes in two flavors isotopes 63Cu and 65Cu 63Cu has an atomic mass of 62 9298 amu and an abundance of 69 09 The other isotope 65Cu has an abundance of 30 91 The average atomic mass between these two isotopes is 63 546 amu
Write the atomic symbol symbol notation for the two isotopes of uranium U whose atomic number is 92 One isotope has 142 neutrons and the other isotope has 146 neutrons 5 Calculate the average atomic mass of the element iron Fe using the following data Isotope 6 Calculate the average atomic mass of the element nitrogen N using the 5 Calculate the relative atomic mass of zinc given that it has five isotopes as shown below Isotope Ir abundance 64Zn 63 93 48 89 66Zn 65 93 27 81 67Zn 66 93 4 11 68Zn 67 93 18 57 70Zn 69 93 0 62 6 Bromine has a relative atomic mass of 79 91 It has two isotopes 79Br of Ir 78 92 and percentage abundance 50 54 and 81Br
More picture related to Calculating Atomic Mass Worksheet

Calculating Average Atomic Mass Worksheet Name Lisa
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/b66a6b64e30394304e6dfcd7fb5d02a1/thumb_1200_1553.png
Atomic Mass And Atomic Number Worksheet Answers Lobo Black Db excel
https://db-excel.com/wp-content/uploads/2019/09/atomic-mass-and-atomic-number-worksheet-answers-lobo-black-750x970.png
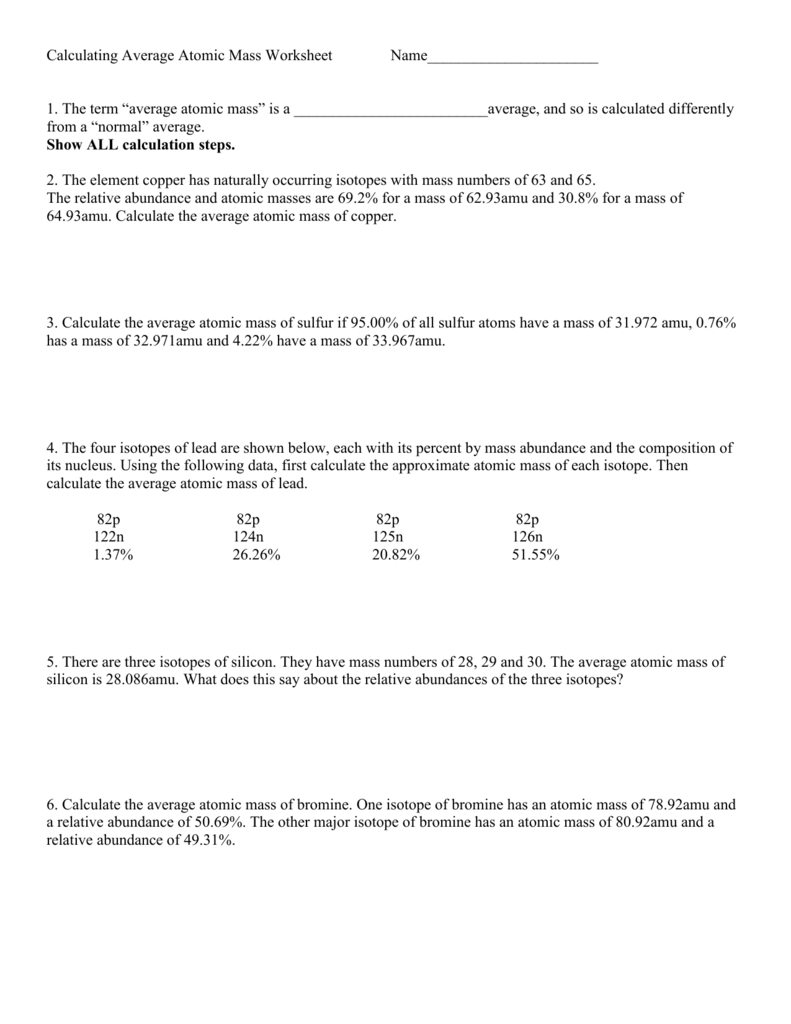
Calculating Average Atomic Mass Worksheet
https://s3.studylib.net/store/data/008665621_1-209339681364457c771ecc1d8305d317.png
PROBLEM 2 3 1 1 2 3 1 1 Determine the number of protons neutrons and electrons in the following isotopes that are used in medical diagnoses a atomic number 9 mass number 18 charge of 1 b atomic number 43 mass number 99 charge of 7 c atomic number 53 atomic mass number 131 charge of 1 Calculations for KS4 Chemistry Worksheet You will need a copy of the Periodic Table to complete these questions 1 Find the Ar relative atomic mass for the following elements Find the Ar relative atomic mass for the following elements a K 39 b F 19 c Mg 24 d O 16 e H 1 f N 14 g Cl 35 5 h Cu 63 5 i S 32 2 Calculate the Mr
Protons Neutrons and Electrons Practice Worksheet Calculating the number of each particle in an atom Protons Atomic Number Electrons Protons Neutrons Atomic Mass Atomic Number OR Big Small Use the periodic table to find the numbers of protons neutrons and electrons for atoms of the following elements Use the atomic masses of each of the two isotopes of chlorine along with their respective percent abundances to calculate the average atomic mass of chlorine Step 1 List the known and unknown quantities and plan the problem Change each percent abundance into decimal form by dividing by 100

Calculating Average Atomic Mass Worksheet Printable Worksheets And
https://i0.wp.com/www.worksheeto.com/postpic/2009/12/average-atomic-mass-and-isotopes-worksheet-answer-key_212396.png?crop=12

Calculating Average Atomic Mass Worksheet Name
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/190b01343490a8257c1a5d2a56d9a2be/thumb_1200_1553.png
Calculating Atomic Mass Worksheet - Pdf 301 43 KB pdf 342 95 KB A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes and percentage abundance Answers provided