Balancing Equations Practice 2 Learn how to balance chemical equations by using the law of conservation of mass and the coefficients of reactants and products Practice with different levels of difficulty and get immediate feedback Compare your results with real life examples and simulations of chemical reactions
The ultimate goal for balancing chemical equations is to make both sides of the reaction the reactants and the products equal in the number of atoms per element This stems from the universal law of the conservation of mass which states that matter can neither be created nor destroyed By Anne Marie Helmenstine Ph D Updated on January 04 2019 A balanced chemical equation gives the number and type of atoms participating in a reaction the reactants products and direction of the reaction
Balancing Equations Practice 2

Balancing Equations Practice 2
https://www.unmisravle.com/wp-content/uploads/2018/04/balancing_equations_practice_worksheet_answers_2.jpg

Balancing Equation Worksheet Fill Online Printable Fillable Blank
https://www.pdffiller.com/preview/269/258/269258541/large.png

Balancing Chemical Equations Practice Worksheet Answer Key Db excel
https://db-excel.com/wp-content/uploads/2019/09/49-balancing-chemical-equations-worksheets-with-answers-101.jpg
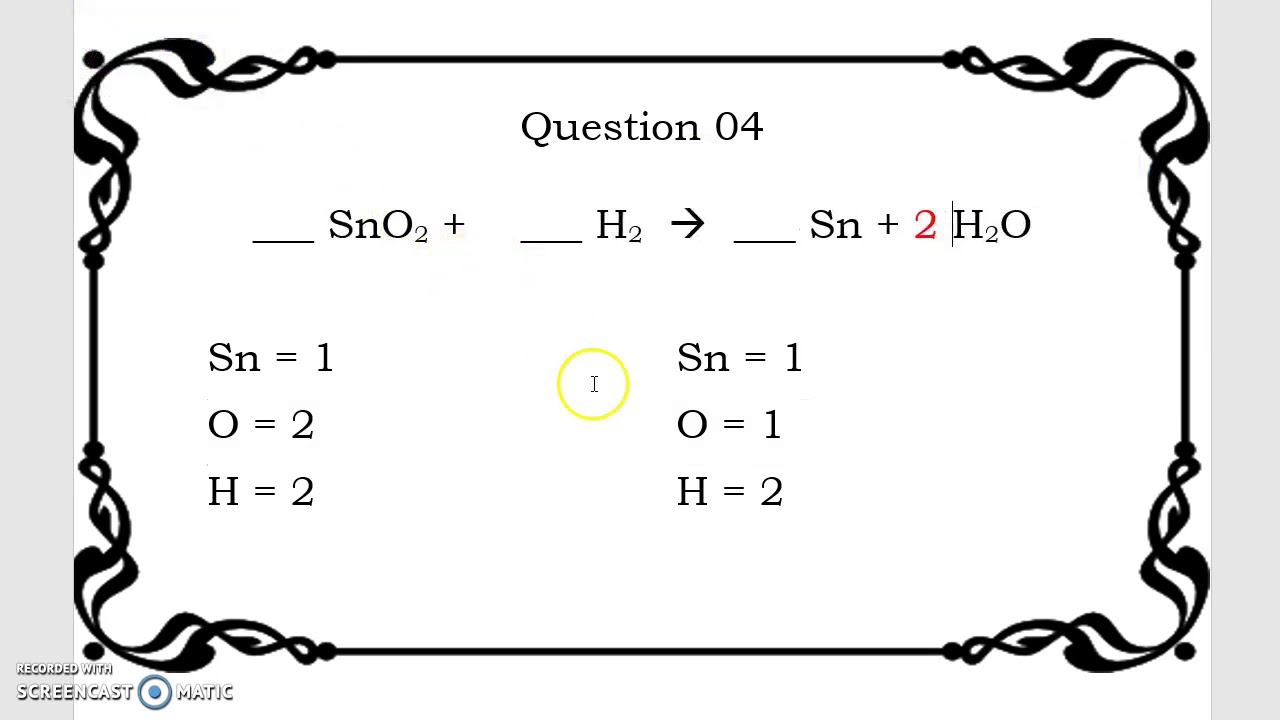
Balancing Equations Practice Quiz Here are 10 unbalanced equations Select the correct balanced equation Take this online quiz to practice balancing chemical equations Vladimir Nenov EyeEm Getty Images By Anne Marie Helmenstine Ph D Updated on October 26 2020 1 SnO H Sn H O 2 SnO 2 H 2 Sn H O Figure 4 2 4 4 2 4 The Relationships among Moles Masses and Formula Units of Compounds in the Balanced Chemical Reaction for the Ammonium Dichromate Volcano Chemical equation N H 4 2 C r 2 O 7 dissociates into C r 2 O 3 N 2 and H 2 O Conversions are given between moles mass and molecules
You might need Calculator The following equation represents the reaction between solid aluminum and hydrochloric acid Balance the equation by filling in the correct coefficients for each substance Al s HCl a q AlCl A 3 a q H A 2 g Show Calculator Stuck Review related articles videos or use a hint Report a problem Balancing Chemical Equations Gap fill exercise Fill in all the gaps then press Check to check your answers Use the Hint button to get a free letter if an answer is giving you trouble You can also click on the button to get a clue Note that you will lose points if you ask for hints or clues C 6 H 6 O 2 CO 2 H 2 O
More picture related to Balancing Equations Practice 2
Balancing Equations Practice Question 04 YouTube
https://i.ytimg.com/vi/3uCG7PEz2S0/maxresdefault.jpg
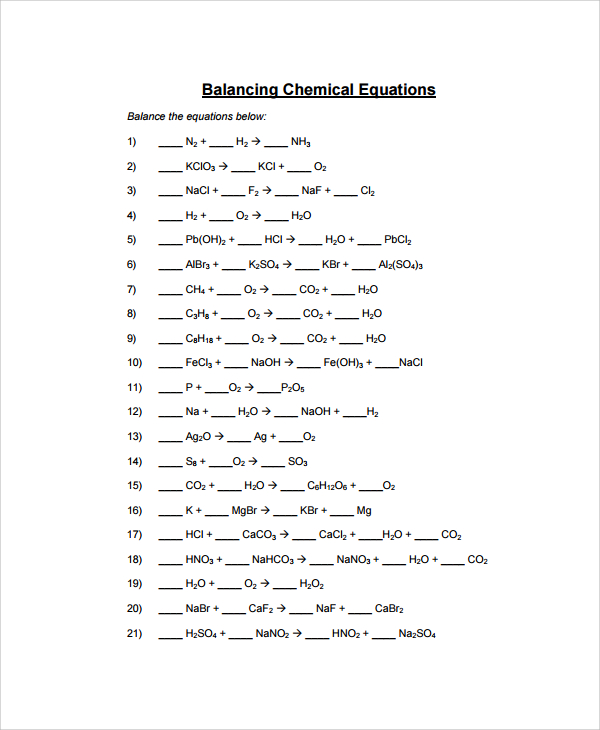
Sample Balancing Equations Worksheet Templates 9 Free Documents
http://images.sampletemplates.com/wp-content/uploads/2016/06/22163500/Simple-Balancing-Equations-Worksheet.jpeg

Balancing Equation Worksheet With Answers
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-08.jpg
How to balance chemical equations We ll start out with examples that show the concepts behind balancing chemical equations We will start with a word equat PROBLEM 5 1 1 7 5 1 1 7 A novel process for obtaining magnesium from sea water involves several reactions Write a balanced chemical equation for each step of the process The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide The second step is the formation of
2 H 2 Sn 2 H 2 O This puts the hydrogen atoms out of balance Now there are two hydrogen atoms on the left and four hydrogen atoms on the right To get four hydrogen atoms on the right add a coefficient of 2 for the hydrogen gas Remember coefficients are multipliers so if we write 2 H 2 O it denotes 2x2 4 hydrogen atoms and 2x1 2 Make sure that all coefficients are in the lowest possible ratio If necessary reduce to the lowest ratio Example 11 3 1 11 3 1 Balancing Chemical Equations Aqueous solutions of lead II nitrate and sodium chloride are mixed The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead II chloride
Balancing Chemical Equations Practice Worksheet Db excel
https://db-excel.com/wp-content/uploads/2019/09/balancing-chemical-equations-practice-sheet-1.png

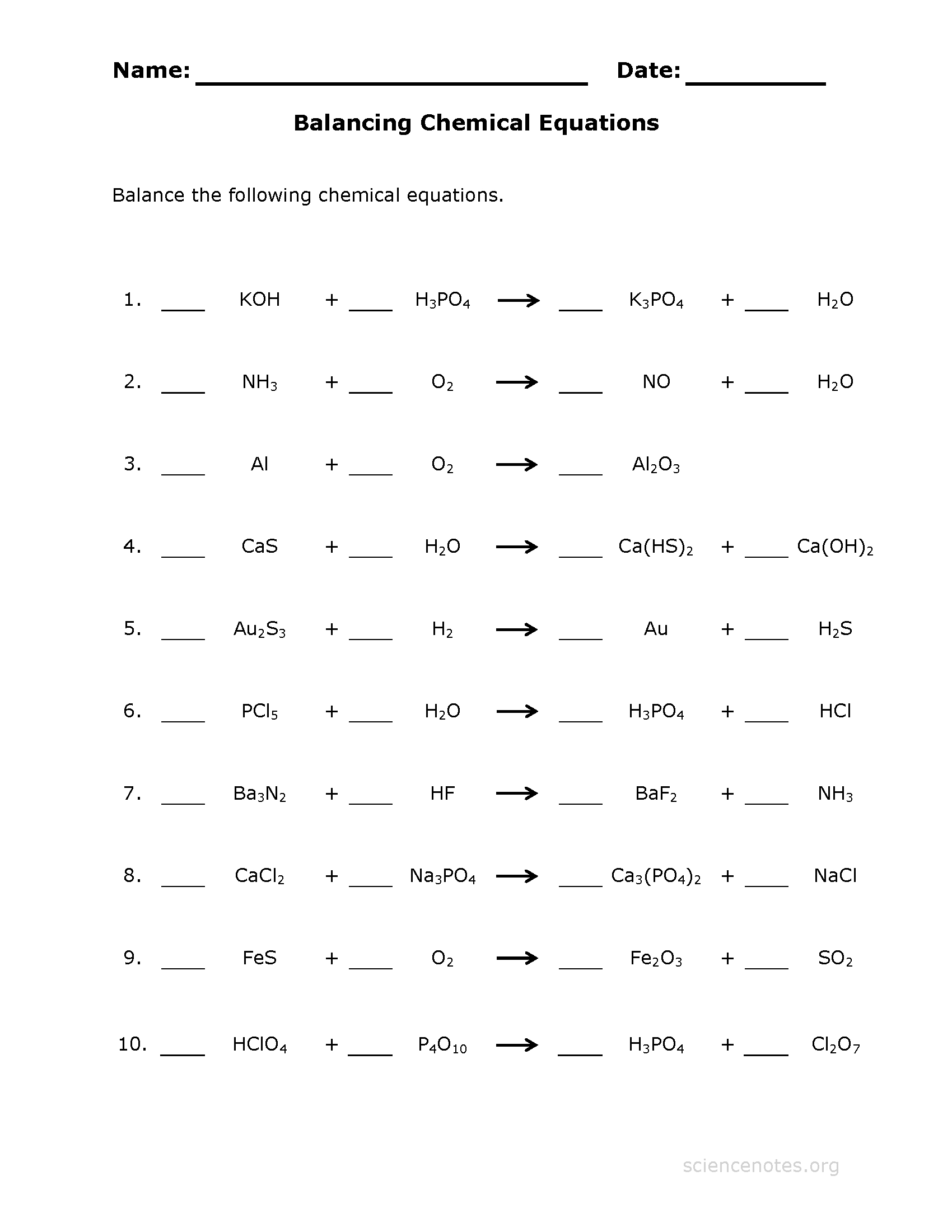
49 Balancing Chemical Equations Worksheets with Answers
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-07-790x1022.jpg
Balancing Equations Practice 2 - Balancing Equations Practice Quiz Here are 10 unbalanced equations Select the correct balanced equation Take this online quiz to practice balancing chemical equations Vladimir Nenov EyeEm Getty Images By Anne Marie Helmenstine Ph D Updated on October 26 2020 1 SnO H Sn H O 2 SnO 2 H 2 Sn H O