Balancing Equations Chemistry Answer Key Balancing chemical equations requires practice Once you ve done it a few times it becomes easier and easier This balancing chemical equations practice sheet has ten more unbalanced chemical equations to solve Download a PDF of this worksheet here A PDF of the answer key is also available here
Balancing Chemical Equations Answer Key Balance the equations below N2 3 H2 2 NH3 KClO3 2 KCl 3 O2 2 NaCl 1 F2 2 NaF 1 Cl2 2 H2 1 O2 2 H2O Pb OH 2 2 HCl 2 H2O 1 PbCl2 AlBr3 3 K2SO4 6 KBr 1 Al2 SO4 3 CH4 2 O2 1 CO2 2 H2O This balancing chemical equations worksheet has ten unbalanced equations to practice your skills Either right click and save the image or else download the PDF of the worksheet here The worksheet prints on a standard sheet of printer paper Balancing Chemical Equations Worksheet Answer Key
Balancing Equations Chemistry Answer Key

Balancing Equations Chemistry Answer Key
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-17.jpg

13 Balancing Equations Worksheet Answer Key Worksheeto
https://www.worksheeto.com/postpic/2015/07/balancing-chemical-equations-worksheet-answers_224100.png
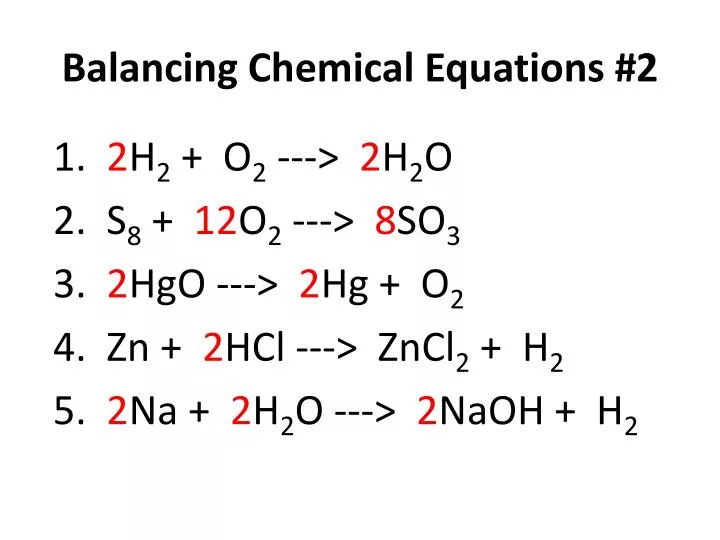
Ideal Balanced Chemical Equation Hcl And Naoh Equations Worksheet With
https://image2.slideserve.com/3871925/balancing-chemical-equations-2-n.jpg
Balance the following chemical equation Mg OH 2 HCl MgCl 2 H 2 O Note All reactants and products require a coefficient of at least one Stuck Review related articles videos or use a hint Report a problem Do 4 problems Figure 4 2 4 4 2 4 The Relationships among Moles Masses and Formula Units of Compounds in the Balanced Chemical Reaction for the Ammonium Dichromate Volcano Chemical equation N H 4 2 C r 2 O 7 dissociates into C r 2 O 3 N 2 and H 2 O Conversions are given between moles mass and molecules
The Key to Balancing Chemical Equations The ultimate goal for balancing chemical equations is to make both sides of the reaction the reactants and the products equal in the number of atoms per element This stems from the universal law of the conservation of mass which states that matter can neither be created nor destroyed A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation We can write a chemical equation for the reaction of carbon with hydrogen gas to form methane CH4 CH 4 C s 2Catoms H2 g 2Hatoms CH4 g 1 Catom 4H atoms C s H 2 g CH 4
More picture related to Balancing Equations Chemistry Answer Key

Balancing Equations Worksheet Answers 1 20
https://i.pinimg.com/originals/25/ff/5b/25ff5bf1d117580dcfb9a9ae4d6b59b2.jpg

Balancing Chemical Equation Worksheet
http://www.unmisravle.com/wp-content/uploads/2018/04/balancing_equations_practice_worksheet_answers_2.jpg

Balancing Chemical Equations Worksheet Answers Pdf Book Lottie Sheets
https://i0.wp.com/www.worksheeto.com/postpic/2012/01/writing-chemical-equations-worksheet-answers-balancing_329457.png
N2 H2 NH3 On the left there is 2 N and 2 H On the right there is 1 N and 3 H If we tried to balance starting with H you d need to use a fraction or decimal and would get messy so let s start with N There s 2 on the left and 1 on the right so we need to change the coefficient of NH3 to 2 Now we have Balancing Chemical Equations Answer Key Balance the equations below 1 1 N2 3 H2 2 NH3 2 2 KClO3 2 KCl 3 O2 3 2 NaCl 1 F2 2 NaF 1 Cl2 4 2 H2 1 O2 2 H2O 5 1 Pb OH 2 2 HCl 2 H2O 1 PbCl2 6 2 AlBr3 3 K2SO4 6 KBr 1 Al2 SO4 3 7 1 CH4 2 O2 1 CO2 2 H2O 8 1 C3H8 5 O2 3 CO2 4 H2O 9 2 C8H18
Example 1 Balancing Chemical Equations Write a balanced equation for the reaction of molecular nitrogen N 2 and oxygen O 2 to form dinitrogen pentoxide reveal answer q 463373 Show Answer reveal answer hidden answer a 463373 First write the unbalanced equation Next count the number of each type of atom present in the Directions First balance each of the chemical equations below Then classify each reaction as synthesis decomposition single replacement or double replacement To earn full credit write the words out when classifying Balance the equation Sb Cl2 SbCl3 Mg O2 MgO

Balancing Chemical Equations Worksheet Answer Key 1 25 Db excel
https://db-excel.com/wp-content/uploads/2019/09/49-balancing-chemical-equations-worksheets-with-answers-131.jpg

Balance Chemical Equations Worksheet 3 Answer Key Science Notes And
http://sciencenotes.org/wp-content/uploads/2015/01/balanceequations3key.png
Balancing Equations Chemistry Answer Key - Balance the equations below N 2 H 2 NH 3 KClO 3 KCl O 2 NaCl F 2 NaF Cl 2 H 2 O 2 H 2 O Pb OH 2 HCl H 2 O PbCl 2 AlBr 3 K 2 SO 4 KBr Al 2 SO 4 3 CH 4 O 2 CO 2 H 2 O