Balancing Equations About Chemistry Answer Key This balancing chemical equations worksheet has ten unbalanced equations to practice your skills Either right click and save the image or else download the PDF of the worksheet here The worksheet prints on a standard sheet of printer paper Balancing Chemical Equations Worksheet Answer Key
Google Classroom Balance the following chemical equation Mg OH 2 HCl MgCl 2 H 2 O Note All reactants and products require a coefficient of at least one Stuck Review related articles videos or use a hint Report a problem Do 4 problems Balancing chemical equations requires practice Once you ve done it a few times it becomes easier and easier This balancing chemical equations practice sheet has ten more unbalanced chemical equations to solve Download a PDF of this worksheet here A PDF of the answer key is also available here
Balancing Equations About Chemistry Answer Key

Balancing Equations About Chemistry Answer Key
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-25.jpg
Balancing Chemical Equations Practice Worksheet With Answers
https://lh5.googleusercontent.com/proxy/R_rfkBkGT8IFYDIsuni9wzwCAubKpe9sTDAGTh01rRv_RvM5xyJj6z9OZd7rt3pUOLFoSntZ2FkGfBEj1wlYCI2ajYDWJ_KoESYu0lRVS3vj5fbEZBS5xsao3SYrFLGfuHncQaRGD0XbGG6xzUfm6ccDs6a9XbE77N6seA=s0-d
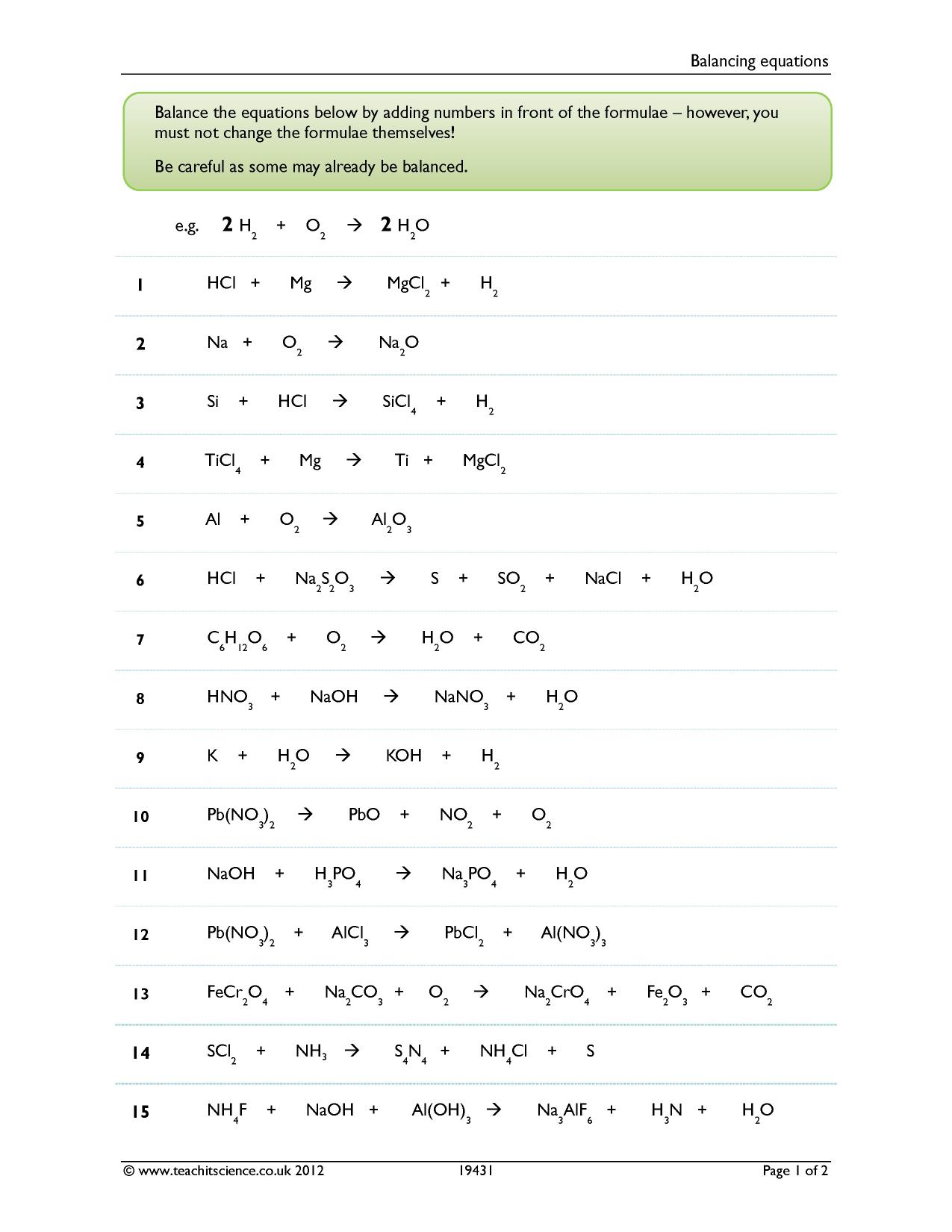
Balancing Equations KS4 Chemistry Teachit
https://www.teachit.co.uk/sites/default/files/products/m_thumbnails/19431/19431_generated.jpeg
The chemical equation described in section 4 1 is balanced meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter Balancing Chemical Equations Answer Key Balance the equations below N2 3 H2 2 NH3 KClO3 2 KCl 3 O2 2 NaCl 1 F2 2 NaF 1 Cl2 2 H2 1 O2 2 H2O Pb OH 2 2 HCl 2 H2O 1 PbCl2 AlBr3 3 K2SO4 6 KBr 1 Al2 SO4 3 CH4 2 O2 1 CO2 2 H2O
A balanced chemical equation shows the same number of each type of atom on both sides of the arrow Questions Tips Thanks Want to join the conversation Sort by Top Voted Gabrielle M 9 years ago I m working on Chemical Reactions Double and Single Replacement on FLVS The ultimate goal for balancing chemical equations is to make both sides of the reaction the reactants and the products equal in the number of atoms per element This stems from the universal law of the conservation of mass which states that matter can neither be created nor destroyed
More picture related to Balancing Equations About Chemistry Answer Key

Balancing Chemical Equation Worksheet
http://www.unmisravle.com/wp-content/uploads/2018/04/balancing_equations_practice_worksheet_answers_2.jpg
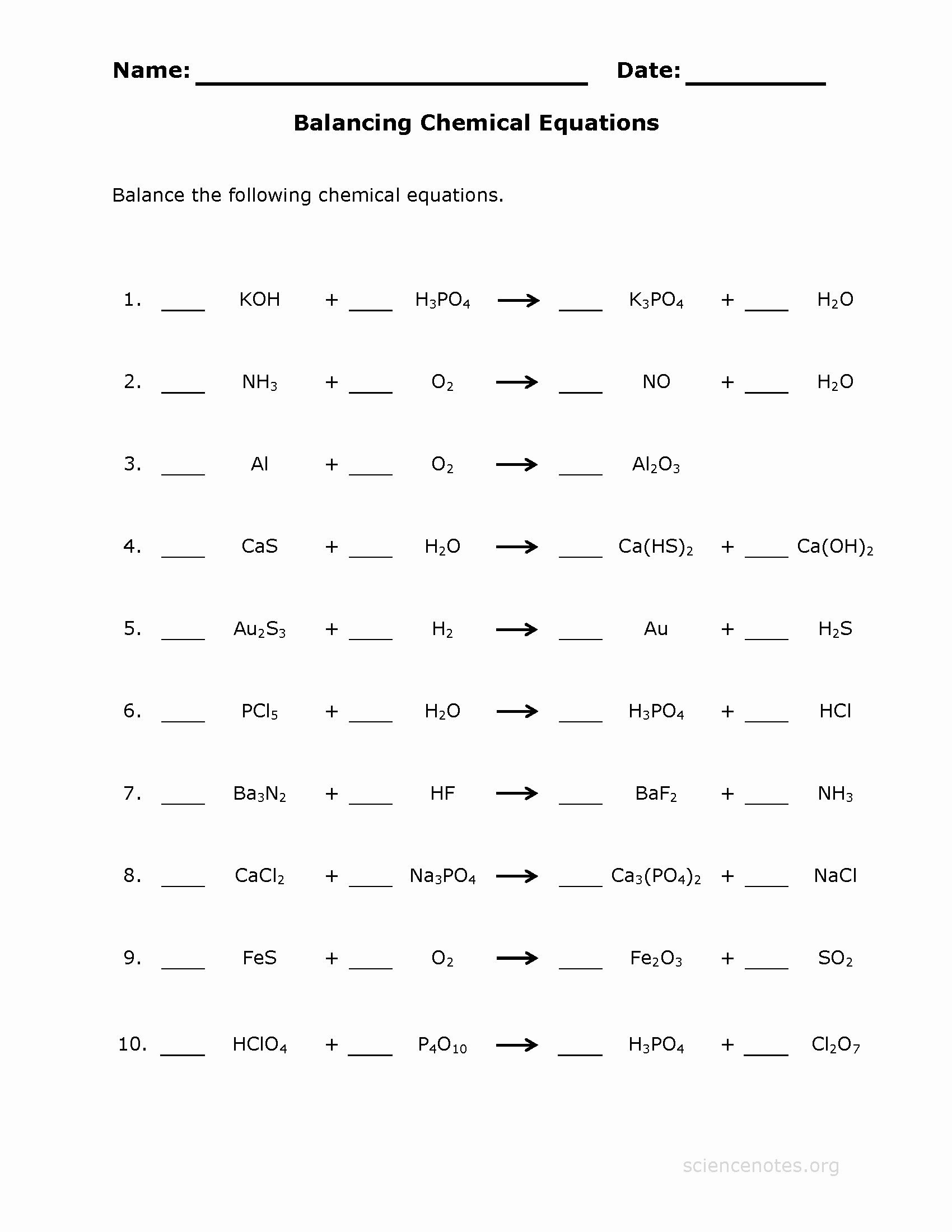
49 Balancing Equation Worksheet With Answers
https://chessmuseum.org/wp-content/uploads/2019/10/balancing-equation-worksheet-with-answers-fresh-balancing-chemical-equations-practice-sheet-of-balancing-equation-worksheet-with-answers.png
Chemistry Balancing Chemical Equations Worksheet Answer Key Pdf Cali
https://i0.wp.com/www.worksheeto.com/postpic/2013/12/chemistry-word-equations-worksheet-answer-key_208079.JPG
Figure 4 2 4 4 2 4 The Relationships among Moles Masses and Formula Units of Compounds in the Balanced Chemical Reaction for the Ammonium Dichromate Volcano Chemical equation N H 4 2 C r 2 O 7 dissociates into C r 2 O 3 N 2 and H 2 O Conversions are given between moles mass and molecules How do you know if a chemical equation is balanced What can you change to balance an equation Play a game to test your ideas
A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation We can write a chemical equation for the reaction of carbon with hydrogen gas to form methane CH4 CH 4 C s 2Catoms H2 g 2Hatoms CH4 g 1 Catom 4H atoms C s H 2 g CH 4 Balance the equation Look at the equation and see which elements are not balanced In this case there are two oxygen atoms on the left side of the equation and only one on the right side Correct this by putting a coefficient of 2 in front of water SnO2 H2 Sn 2 H2O This puts the hydrogen atoms out of balance

Balance Chemical Equations Worksheet 3 Answer Key Science Notes And
http://sciencenotes.org/wp-content/uploads/2015/01/balanceequations3key.png

49 Balancing Chemical Equations Worksheets with Answers
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-17.jpg
Balancing Equations About Chemistry Answer Key - The ultimate goal for balancing chemical equations is to make both sides of the reaction the reactants and the products equal in the number of atoms per element This stems from the universal law of the conservation of mass which states that matter can neither be created nor destroyed