Balancing Chemical Equations Phet Answers Learn how to balance chemical equations by using the law of conservation of mass and the coefficients of reactants and products Practice with different levels of difficulty and get immediate feedback Compare your results with real life examples and simulations of chemical reactions
Explain your answer For each balanced reaction indicate the total number of atoms in the table below Is the number of total atoms on the left side of a balanced equation always equal to the number of total atoms on the right side of the equation As a group play level 1 of the balancing equation game Balancing Chemical Equations PhET Interactive Simulations
Balancing Chemical Equations Phet Answers

Balancing Chemical Equations Phet Answers
https://i2.wp.com/db-excel.com/wp-content/uploads/2019/09/49-balancing-chemical-equations-worksheets-with-answers-130.jpg

Balancing Equation Worksheet With Answers
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-08.jpg

Phet Balancing Chemical Equations Game Answer Key
https://i2.wp.com/i.ytimg.com/vi/Os7Vh5wd12s/maxresdefault.jpg
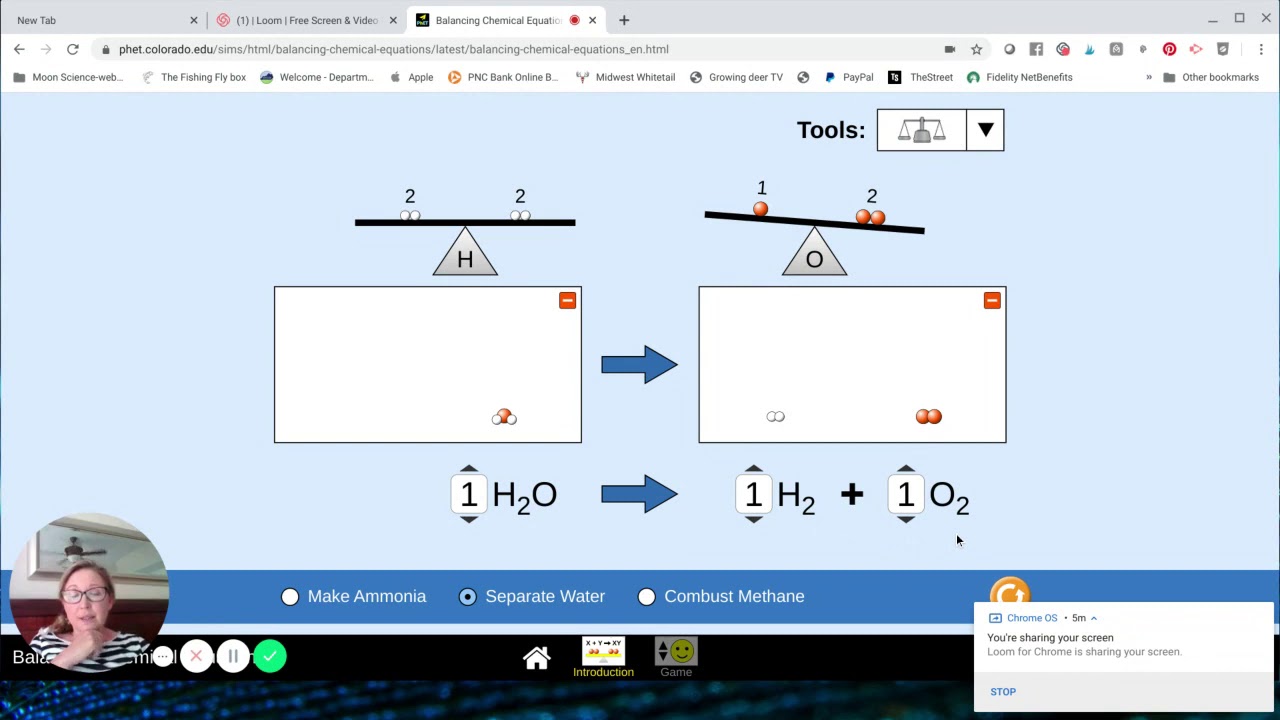
Description How do you know if a chemical equation is balanced What can you change to balance an equation Play a game to test your ideas Sample Learning Goals Balance a chemical equation Recognize that the number of atoms of each element is conserved in a chemical reaction Balancing Chemical Equations 30 Favorites Balancing Chemical Equations 30 Favorites SIMULATION in Balancing Equations Conservation of Mass Last updated March 15 2022 The simulation for September comes from PhET AACT helped fund the conversion of this popular simulation into a format that is compatible with all devices including iPads
Chemistry in Context December 2 2019 Credit PhET Interactive Simulations View Full Screen This interactive activity allows you to practice balancing equations and offers a game to check your understanding Related Tags Reactions This interactive activity allows you to practice balancing equations and offers a game to check your understanding Sample Learning Goals Balance a chemical equation Recognize that the number of atoms of each element is conserved in a chemical reaction Describe the difference between coefficients and subscripts in a chemical equation Translate from symbolic to molecular representations of matter Version 1 1 9 For Teachers Teacher Tips
More picture related to Balancing Chemical Equations Phet Answers
PHET Balancing Equations Simulation YouTube
https://i.ytimg.com/vi/GCtK61mFqfs/maxresdefault.jpg

Phet Balancing Chemical Equations Worksheet Answers
https://i2.wp.com/s3.studylib.net/store/data/008927293_1-3bd2d0f80acddf368e4625addf818c11.png
Solved Andrew Nordstrom PHET Balancing Chemical Equations Part 1
https://www.coursehero.com/qa/attachment/16372008/
Balance the following chemical equation Mg OH 2 HCl MgCl 2 H 2 O Note All reactants and products require a coefficient of at least one Stuck Review related articles videos or use a hint Balancing Chemical Equations phet dev colorado edu
CO2 H2O C6H12O6 O2 The first step to balancing chemical equations is to focus on elements that only appear once on each side of the equation Here both carbon and hydrogen fit this requirement So we will start with carbon There is only one atom of carbon on the left hand side but six on the right hand side Balancing equations using a visual representation of particles Checking results to verify that a chemical equation is correctly balanced This worksheet collection is four 4 pages long and consists of Two 2 pages of questions diagrams involving the PhET simulator One 1 full page of balancing questions using the simulator to check results
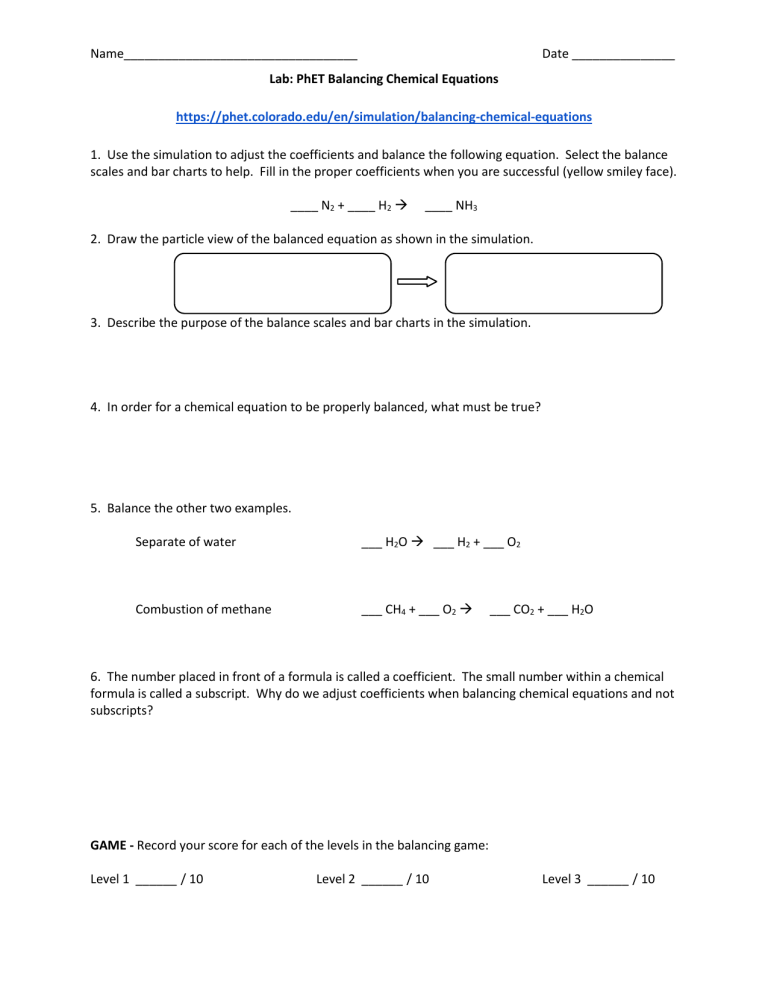
Lab PhET Balancing Chemical Equations
https://s3.studylib.net/store/data/025234792_1-831d19cbfbba80d6e20327fc570ae229-768x994.png

49 Balancing Chemical Equations Worksheets with Answers
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-07-790x1022.jpg
Balancing Chemical Equations Phet Answers - Sample Learning Goals Balance a chemical equation Recognize that the number of atoms of each element is conserved in a chemical reaction Describe the difference between coefficients and subscripts in a chemical equation Translate from symbolic to molecular representations of matter Version 1 1 9 For Teachers Teacher Tips