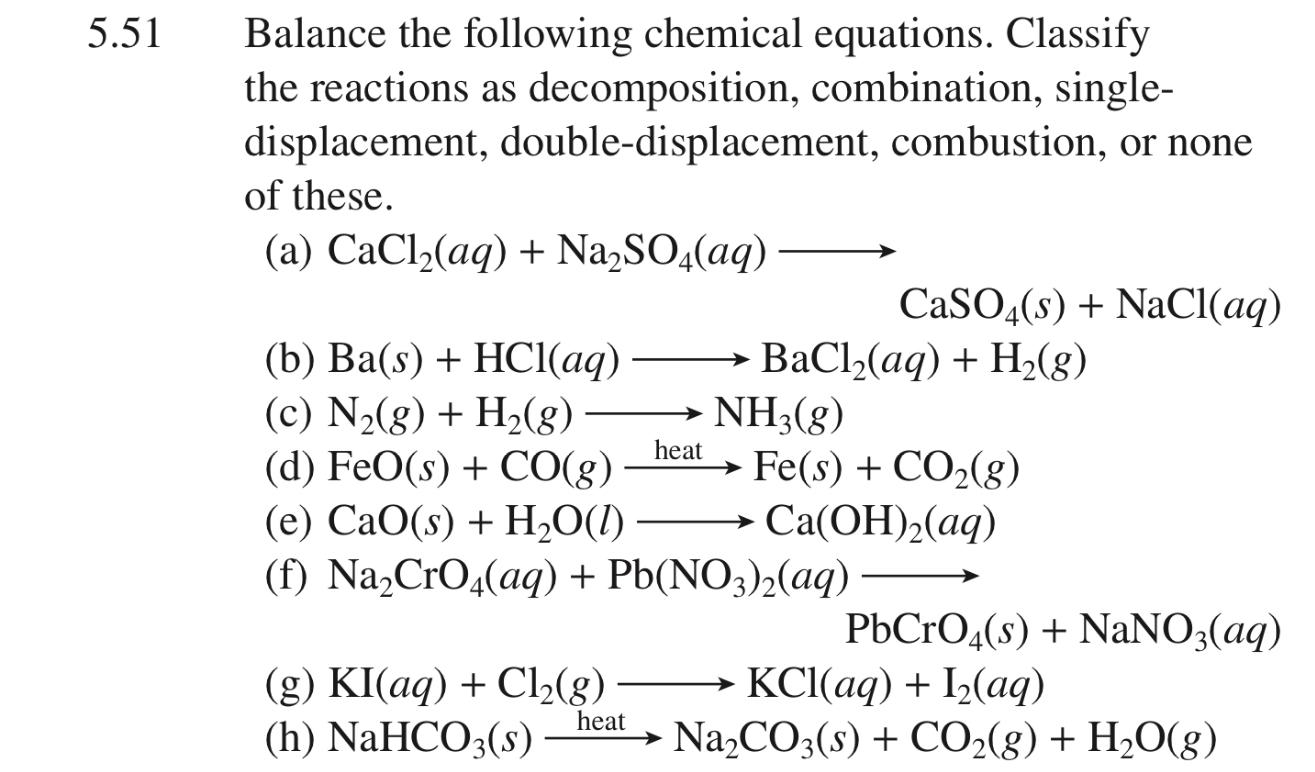
Balance Each Of The Following Skeletal Equations Balance each of the following skeletal equations a Al2O3 s HCl aq AlCl3 aq H2O l b PCl3 l AgF s PF3 g AgCl s c NO2 g NO g O2 g This problem has been solved You ll get a detailed solution from a subject matter expert that helps you learn core concepts See Answer
Robert Carter University of Massachusetts Boston Table of contents Oxidation reduction reactions also called redox reactions involve the transfer of electrons from one species to another These kinds of reactions are at the heart of energy producing devices such as batteries and fuel cells Balance each of the following skeletal equations by using oxidation and reduction half reactions All the reactions take place in basic solution Identify the oxidizing agent and reducing agent in each reaction Production of chlorite ions from dichlorine heptoxide Cl2O7 g H2O2 aq ClO2 aq O2 g
Balance Each Of The Following Skeletal Equations
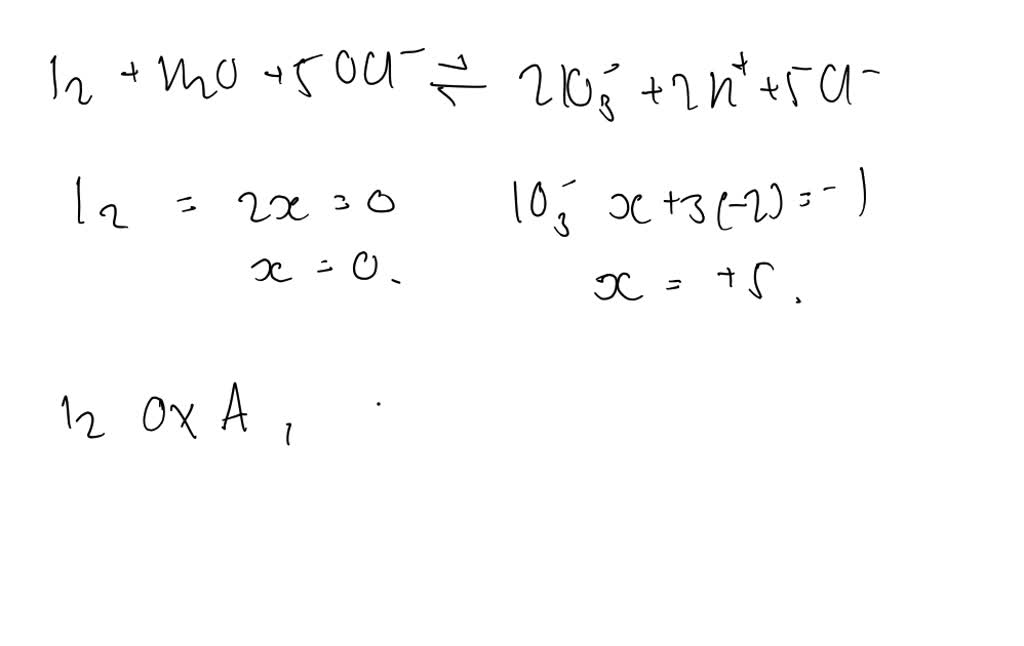
Balance Each Of The Following Skeletal Equations
https://cdn.numerade.com/previews/eea7afec-b6d7-4972-9302-a89252e43c04_large.jpg
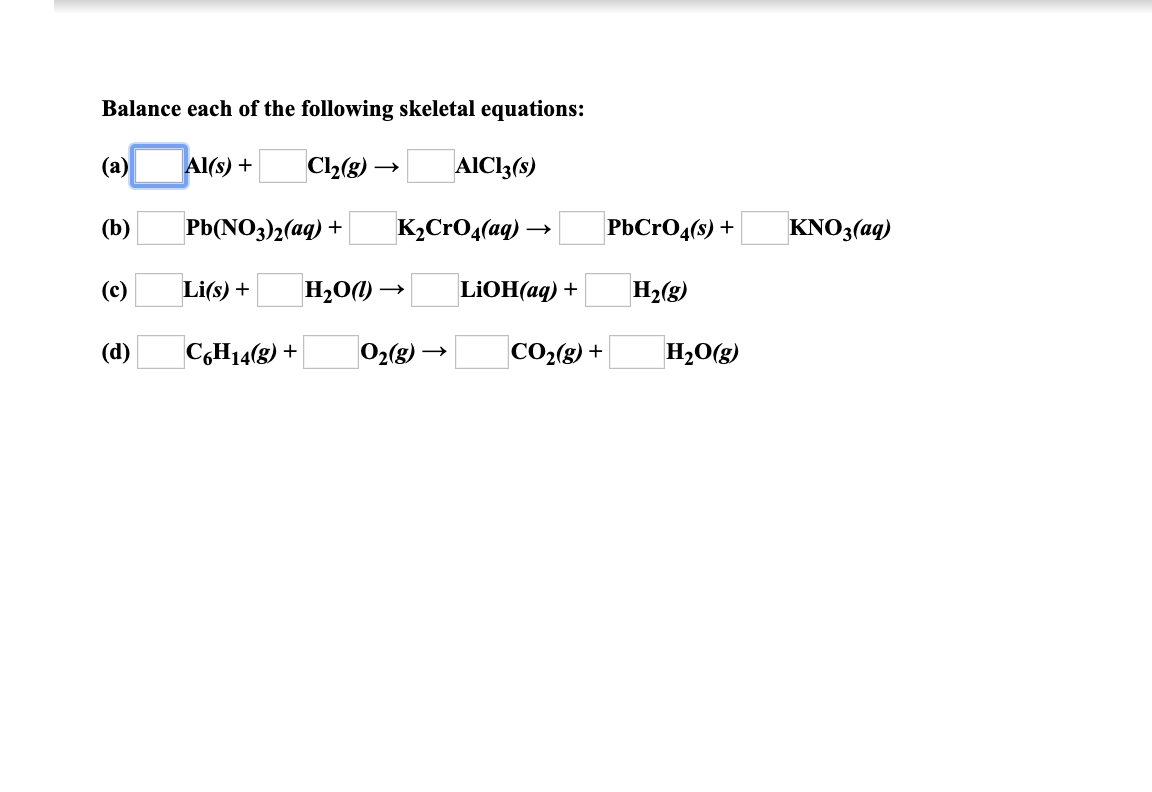
Solved Balance Each Of The Following Skeletal Equations a Chegg
https://media.cheggcdn.com/media/af2/af27158e-120d-45ac-b7a7-00c64211b349/phpcDrhma.png
Solved Balance Each Of The Following Skeletal Equations b Chegg
https://media.cheggcdn.com/media/357/35757bb3-5acf-4e9e-965d-a75427b5a692/phpg692Ya
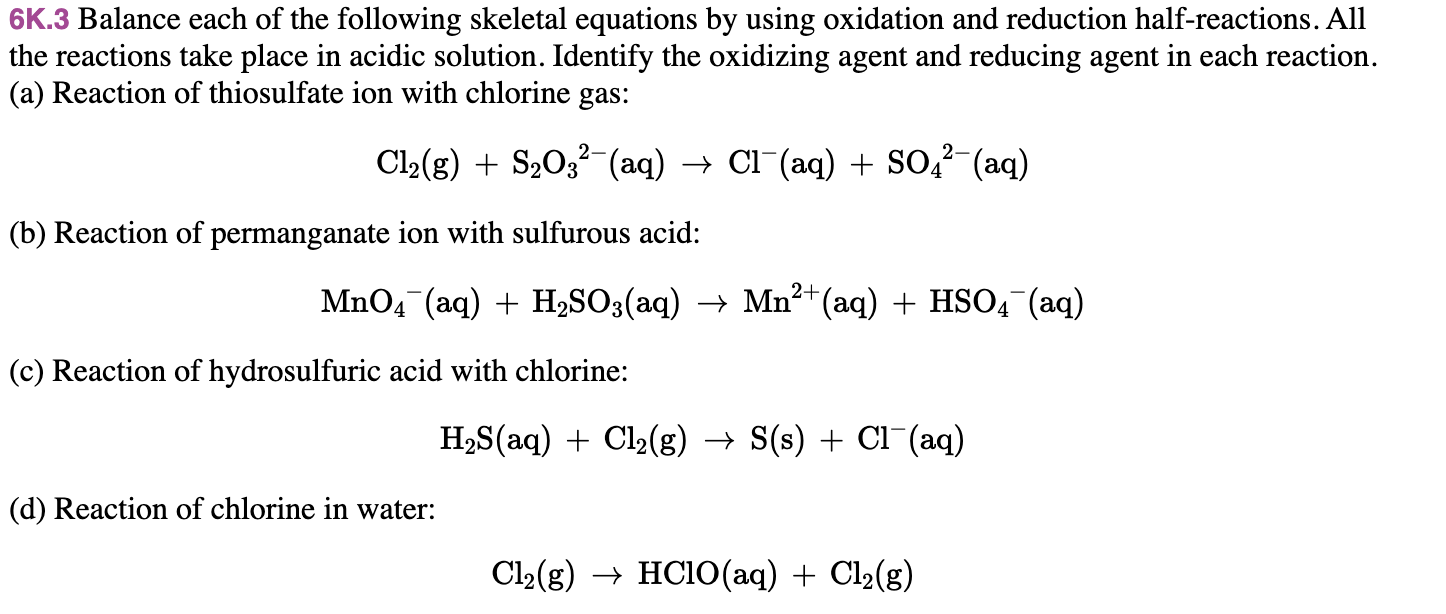
6k 3 d Identifying Half Reactions Balance each of the following skeletal equations by using oxidation and reduction half reactions All the reactions take place in acidic solution Identify the oxidizing agent and reducing agent in each reaction I understand that Cl2 is oxidized to form HClO but I can t figure out the second half reaction In these cases equations representing the redox reaction can be very challenging to balance by inspection and the use of a systematic approach called the half reaction method is helpful This approach involves the following steps Write skeletal equations for the oxidation and reduction half reactions
To balance a redox equation by the ion electron method carry out the following steps in this sequence 1 Separate the skeletal equation into two half reactions One half reaction will be a reduction and the other will be an oxidation It is not necessary at this stage to identify which is which 2 Balance each half reaction separately To balance an equation count first the number of atoms in both the reactants and the products side and put necessary numerical coefficients before each element that need to be balanced To check if the equation is balanced count the total number of atoms for the reactants and products side for each element by multiplying the numerical coefficients with the corresponding subscript on the
More picture related to Balance Each Of The Following Skeletal Equations

Balance The Following Chemical Equation Brainly in
https://hi-static.z-dn.net/files/d36/ffe33750136eb699abc8e4a3490e431c.jpg
Solved 6 K 3 Balance Each Of The Following Skeletal Chegg
https://media.cheggcdn.com/media/84f/84fbeb15-5cc3-4bd1-847b-4b9fd215b486/phpp77PHW

Spice Of Lyfe Chemical Equation And Reaction Class 10 Questions
https://www.wikihow.com/images/6/63/Balance-Chemical-Equations-Step-7Bullet3-Version-2.jpg
6K 5 Balance each of the following skeletal equations by using oxidation and reduction half reactions All the reactions take place in basic solution Identify the oxidizing agent and reducing agent in each reaction a Reaction of ozone with bromide ions O3 aq Br aq O2 g BrO3 aq Balance each of the following skeletal equations by using oxidation and reduction half reactions All the reactions take place in acidic solution Identify the oxidizing agent and reducing agent in each reaction a Reaction of thiosulfate ion with chlorine gas Cl2 g S2O3 2 aq Cl aq SO4 2 aq
A chemical equation is said to be balanced if the number of atoms of each element on the left hand side is equal to the number of atoms present on the right hand side In order to balance a chemical equation it is required to know what are reactants that are taking part in a chemical reaction and what are products will be formed Step 1 Plan the problem Follow the steps for writing and balancing a chemical equation Step 2 Solve Write the skeleton equation with the correct formulas Pb NO3 2 aq NaCl aq NaNO3 aq PbCl2 s Count the number of each atom or polyatomic ion on both sides of the equation The nitrate ions and the chlorine atoms are unbalanced

Q 10 Balance The Following Skeletal Equations Brainly in
https://hi-static.z-dn.net/files/d26/a6a25da748a936004813ef9d23aaf637.jpg

Q 10 Balance The Following Skeletal Equations Brainly in
https://hi-static.z-dn.net/files/d81/8a685c711f2df1bf4b6d4df8cbb80056.jpg
Balance Each Of The Following Skeletal Equations - This problem has been solved You ll get a detailed solution from a subject matter expert that helps you learn core concepts See Answer Question Balance each of the following skeletal equations a L AI s 1 C12 g L AICI3 s d C6H14 g 1 2 g CO2 g H2O g Show transcribed image text Expert Answer Step 1