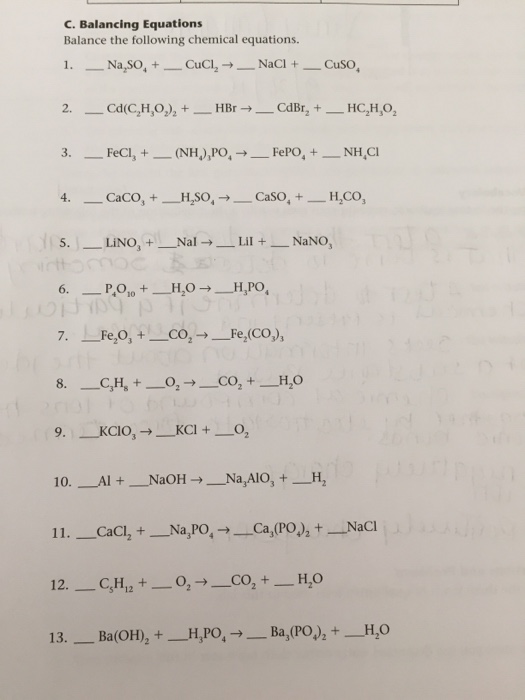Balance Each Of The Following Equations Instructions on balancing chemical equations Enter an equation of a chemical reaction and click Balance The answer will appear below Always use the upper case for the first character in the element name and the lower case for the second character Examples Fe Au Co Br C O N F Compare Co cobalt and CO carbon monoxide
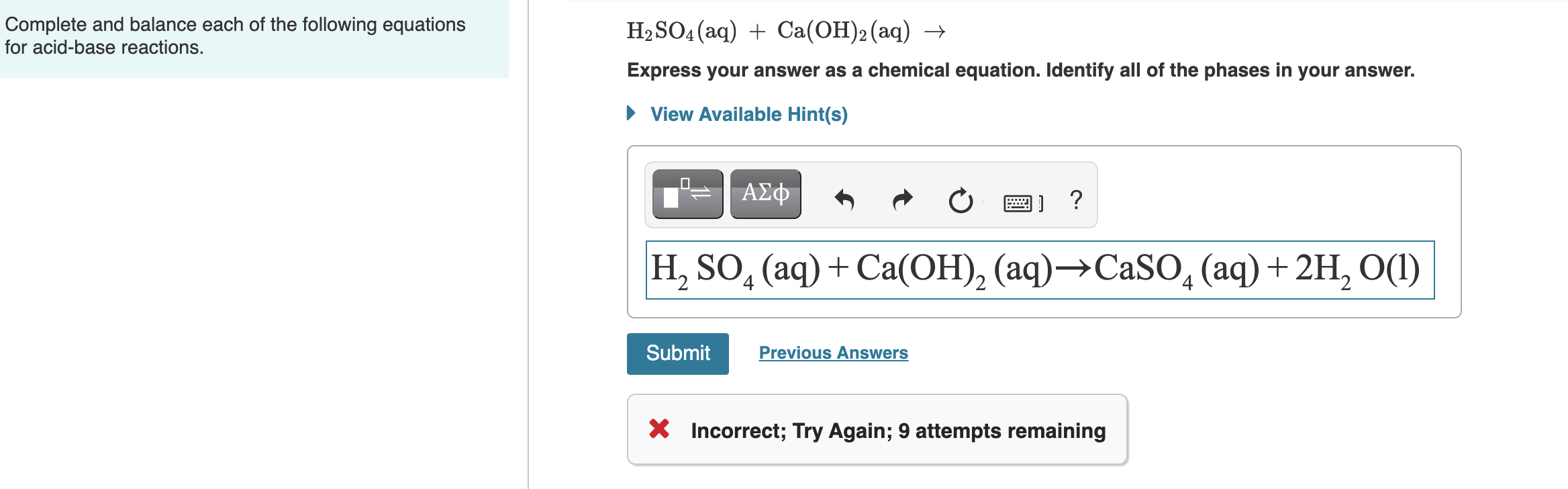
The chemical equation described in section 4 1 is balanced meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter It may be confirmed by simply summing the numbers of Balance each of the following chemical equations A Mg s Br2 l MgBr2 s Express your answer as a chemical equation Identify all of the phases in your answer B P4 s O2 g P4O10 s Express your answer as a chemical equation Identify all of the phases in your answer C Ba OH 2 aq HNO3 aq Ba NO3 2 aq H2O l
Balance Each Of The Following Equations

Balance Each Of The Following Equations
https://media.cheggcdn.com/media/762/762467bd-faf0-464d-be77-97ce455eb7d4/phpmPcxWD.png
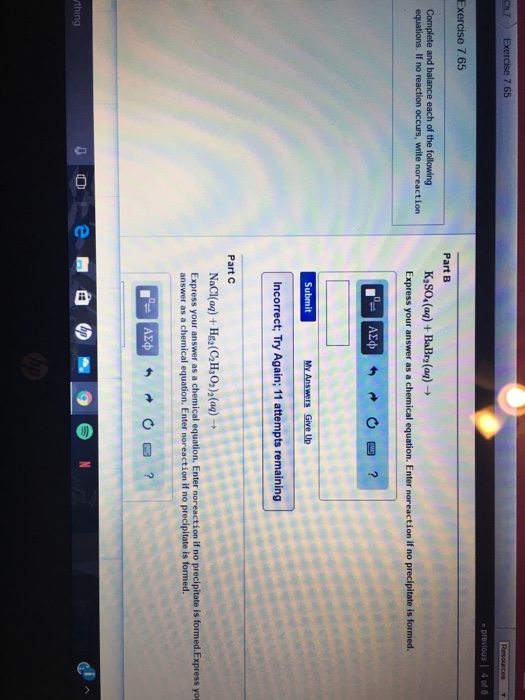
Solved Complete And Balance Each Of The Following Equations Chegg
https://media.cheggcdn.com/media/b1d/b1d22c81-faf1-4168-bcbe-1b2efb1e89ab/phpeQOEgv.png
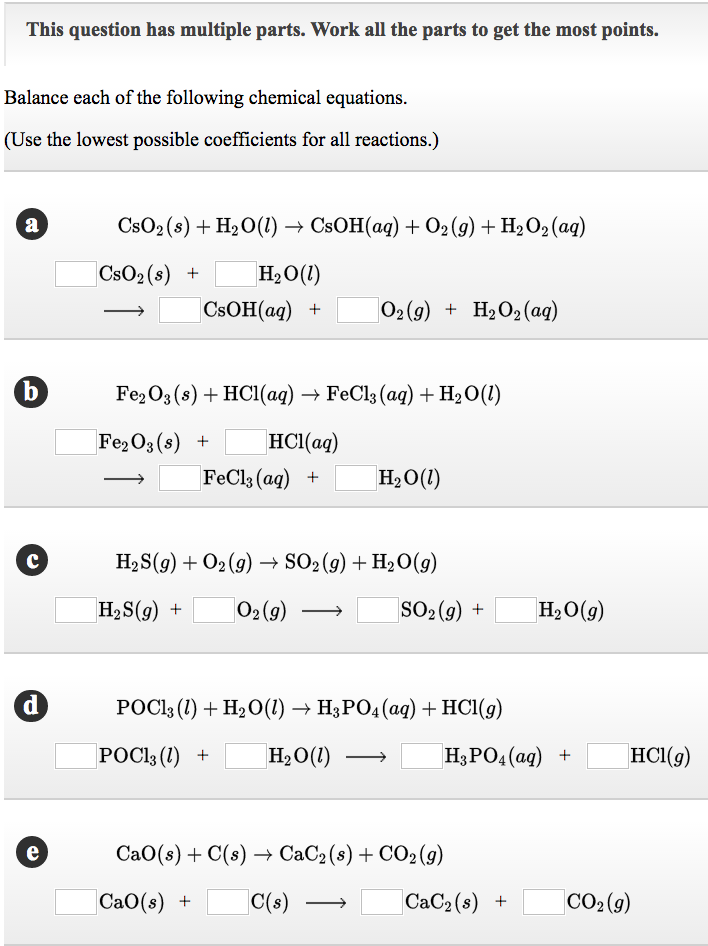
Solved Balance Each Of The Following Chemical Equations Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/dfc/dfcb8f88-5a5d-4f9e-8403-8433df52d1e8/phpNZOpXQ.png
Balance each of the following equations Balancing Equations Answers to Practice Problems Balanced equations Coefficients equal to one 1 do not need to be shown in your answers 2 Fe 3 Cl2 2 FeCl3 4 Fe 3 O2 2 Fe2O3 2 FeBr 3 3 H2SO4 1 Fe2 SO4 3 d 1 C4H6O3 1 H2O 2 C2H4O2 e 1 C2H4 3 O2 2 CO2 2 H2O Balance a chemical equation when given the unbalanced equation Explain the role of the Law of Conservation of Mass in a chemical reaction Even though chemical compounds are broken up and new compounds are formed during a chemical reaction atoms in the reactants do not disappear nor do new atoms appear to form the products
Example 1 Balance the following equation H 2 O 2 H 2 O It is an unbalanced equation sometimes also called a skeleton equation This means that there are UNEQUAL numbers at least one atom on each side of the arrow By the way a skeleton equation is not wrong it just hasn t been balanced yet Presenting it as being balanced would be To balance a redox equation using the half reaction method the equation is first divided into two half reactions one representing oxidation and one representing reduction The equations for the half reactions are then balanced for mass and charge and if necessary adjusted so that the number of electrons transferred in each equation is the same
More picture related to Balance Each Of The Following Equations
Solved Balance The Following Chemical Equations Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/1eb/1eb1a1f2-6fe4-4444-afe5-b8a1e5a873c1/image

How To Balance Chemical Equations 11 Steps with Pictures
https://www.wikihow.com/images/6/63/Balance-Chemical-Equations-Step-7Bullet3-Version-2.jpg
Solved 1 Balance Each Of The Following Equations a Fe Chegg
https://media.cheggcdn.com/study/aa3/aa343ad8-58bc-4e93-941e-fb252fb7da19/image
A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation We can write a chemical equation for the reaction of carbon with hydrogen gas to form methane CH4 CH 4 C s 2Catoms H2 g 2Hatoms CH4 g 1 Catom 4H atoms C s H 2 g CH 4 Balancing Equations The chemical equation described above in Figure PageIndex 1 is balanced meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter
The equations for each element are listed together to form a system of equations In this example the system of equations is as follows Using the algebraic method of balancing chemical equations the following variables can be assigned to the unbalanced equation aAl bO 2 cAl 2 O 3 The equation for Aluminum a 2c The equation Balance each of the following equations and classify each of the reactions by type 1 KClO3 KCl O2 2 Na O2 Na2O2 3 KI Pb NO3 2 KNO3 PbI2 4 Cu AgNO3 Cu NO3 2 Ag 5 C3H8 O2 CO2 H2O Heat 6 Zn HCl ZnCl2 H2 7
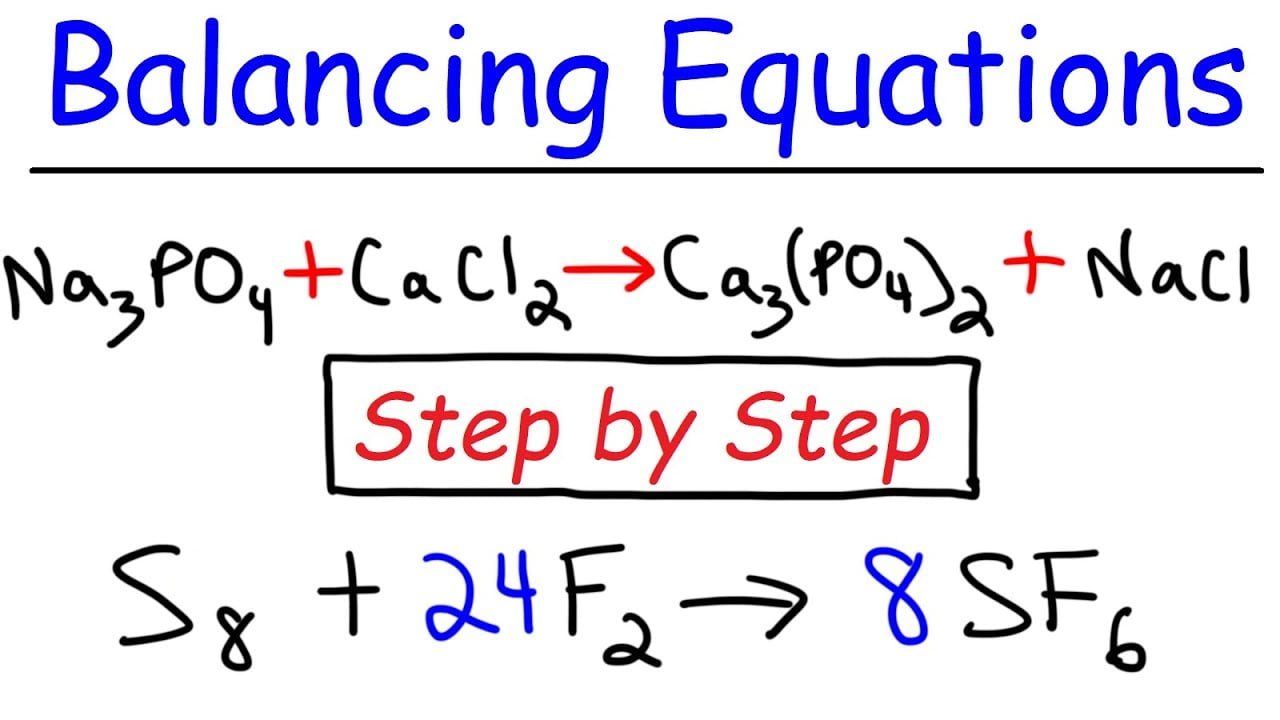
Easy Way Of Balancing Chemical Equations And Exercise Solutions
https://nkedugists.com.ng/wp-content/uploads/2020/05/Balancing-of-chemical-equation.jpg
Solved Complete And Balance Each Of The Following Equations Chegg
https://media.cheggcdn.com/media/2d3/2d39c228-568a-403d-83e9-3413b3b5648c/image
Balance Each Of The Following Equations - To balance a redox equation using the half reaction method the equation is first divided into two half reactions one representing oxidation and one representing reduction The equations for the half reactions are then balanced for mass and charge and if necessary adjusted so that the number of electrons transferred in each equation is the same