Average Atomic Mass Calculations Worksheet Average Atomic Mass Worksheet show all work 1 Rubidium is a soft silvery white metal that has two common isotopes 85Rb and 87Rb If the abundance of 85Rb is 72 2 and the abundance of 87Rb is 27 8 what is the average atomic mass of rubidium 2 Uranium is used in nuclear reactors and is a rare element on earth
Use the atomic masses of each of the two isotopes of chlorine along with their respective percent abundances to calculate the average atomic mass of chlorine Step 1 List the known and unknown quantities and plan the problem Change each percent abundance into decimal form by dividing by 100 Use the equation in question 1 to calculate the atomic mass of an element that has two isotopes each with 50 00 abundance One isotope has a mass of 63 00 amu and the other has a mass of 68 00 amu b Recalculate the atomic mass if instead there is 80 00 of the 63 00 amu isotope and 20 00 of the 68 00 amu isotope
Average Atomic Mass Calculations Worksheet
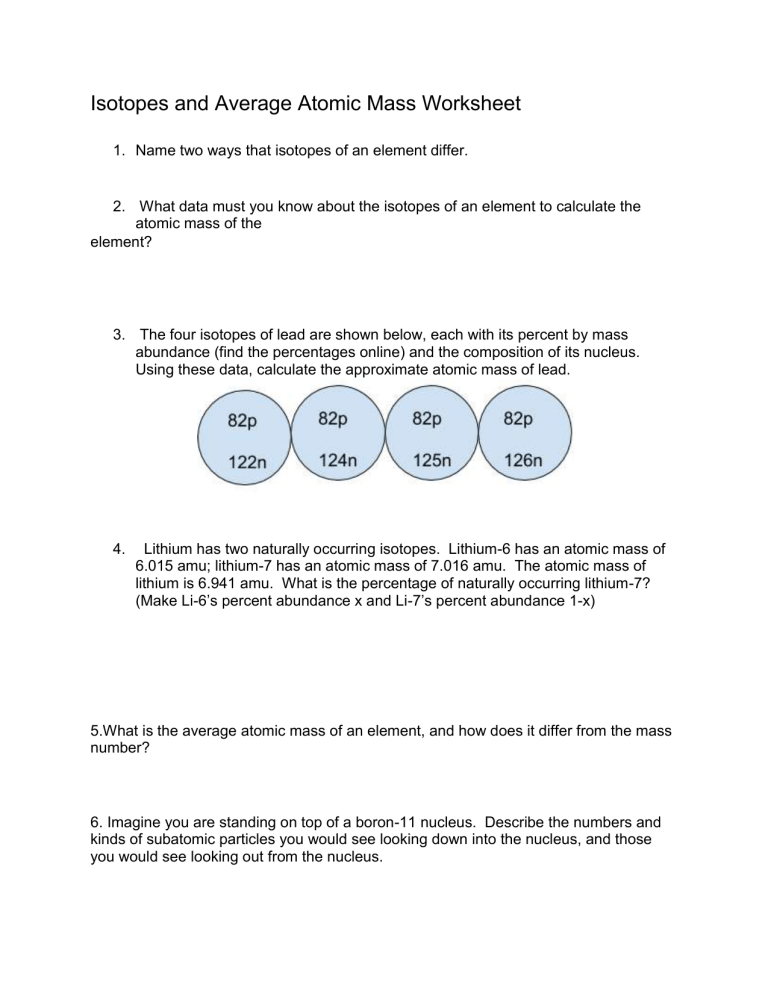
Average Atomic Mass Calculations Worksheet
https://s3.studylib.net/store/data/025390754_1-22e3485f287ae4d47db74f7062afad04-768x994.png
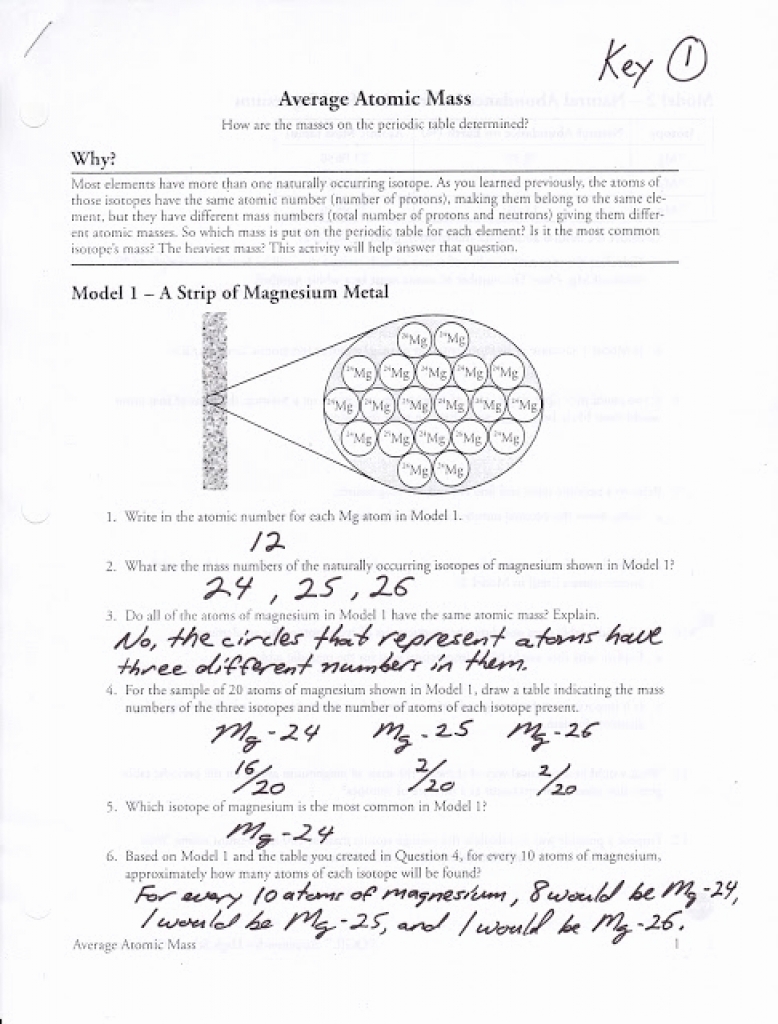
Calculating Average Atomic Mass Worksheet
https://www.unmisravle.com/wp-content/uploads/2018/03/average_atomic_mass_worksheet_answer_key_1.jpg

Average Atomic Mass Worksheet
https://db-excel.com/wp-content/uploads/2019/09/review-worksheet-keys-728x942.png
Calculate the average atomic mass of the element nitrogen N using the following data Isotope abundance Nitrogen 14 95 Nitrogen 15 3 Nitrogen 16 2 7 Calculate the average atomic mass of the element Iodine I using the following data Isotope abundance Iodine 127 80 Iodine 126 17 Iodine 128 3 8 To calculate average atomic mass of an element Average atomic mass fractional abundance of isotope 1 atomic mass of isotope 1 fractional abundance of isotope 2 atomic mass of isotope 2 Practice Problems 1 Chlorine has two isotopes Chlorine 35 has an actual mass of 34 9689 u and chlorine 37 has a mass of 36 9659 u
1 PRACTICE PROBLEM How does an element s atomic mass differ from its atomic number 2 PRACTICE PROBLEM Initially the atomic mass unit was defined as 1 16 of the atomic mass of oxygen atomic mass of O 16 amu Determine the mass of a 14 N atom using the old definition if the current atomic mass of oxygen is 15 994 amu Exercise 2 3 1 2 3 1 A fictional element has two isotopes and an atomic mass of 131 244 amu If the first isotope Isotope 1 has a mass of 129 588amu and the second isotope Isotope 2 has a mass of 131 912 amu which isotope has the greatest natural abundance A Isotope 1 B Isotope 2 C There are equal amounts
More picture related to Average Atomic Mass Calculations Worksheet

Average Atomic Mass Worksheet Solutions
https://s3.studylib.net/store/data/007575700_2-5b782c17a95e8dd3410979aee126d8c7-768x994.png

Average Atomic Mass Worksheet
https://s3.studylib.net/store/data/025416346_1-d3b8ed0a4ef8f6a31cee725cfb240743-768x994.png
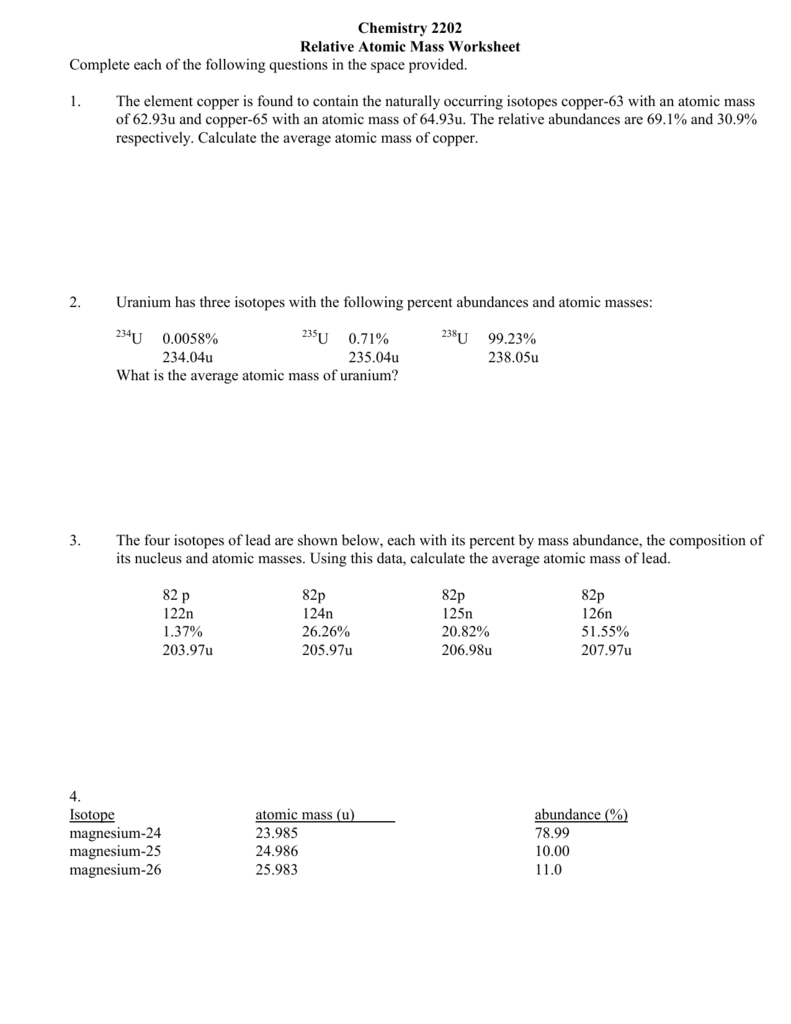
Average Atomic Mass Worksheet
https://s3.studylib.net/store/data/008342063_1-db5fd2c98d3957944061536c714386ef.png
Calculate the average atomic mass of sulfur if 95 00 of all sulfur atoms have a mass of 31 972 amu 0 76 has a mass of 32 971amu and 4 22 have a mass of 33 967amu 4 Calculate the average atomic mass of bromine One isotope of bromine has an atomic mass of 78 92amu and a relative abundance of 50 69 Average Atomic Mass 2 worksheet Live Worksheets Home Worksheets Average Atomic Mass 2 Average Atomic Mass 2 jlinde Member for 1 year 2 months Age 15 Level 10 Language English en ID 2231499 01 12 2022 Country code US Country United States School subject Chemistry 1061818 Main content Atomic Mass 2104157
The relative abundance of Deutrium 1 proton 1 neutron is so small that it is barely accounted for when calculating the average atomic mass So the mass of a proton is around 1 008 amu not 1 Following this logic the carbon 12 atom s atomic mass should actually be 12 09 amu however the mass is exactly 12 amu 4 Naturally occurring strontium consists of four isotopes Sr 84 Sr 86 Sr 87 and Sr 88 Below is the data concerning strontium What is the average atomic mass for Strontium Calculate the average atomic mass of bromine a relative abundance of 50 69 The other major isotope of bromine has an atomic mass of 80 92u and a

Calculating Average Atomic Mass Worksheet Printable Worksheets And
https://i0.wp.com/www.worksheeto.com/postpic/2009/12/average-atomic-mass-and-isotopes-worksheet-answer-key_212396.png?crop=12

Average Atomic Mass Worksheet Db excel
https://db-excel.com/wp-content/uploads/2019/09/average-atomic-mass-worksheet-3.png
Average Atomic Mass Calculations Worksheet - Average atomic mass worksheet Live Worksheets Search Worksheets Home Worksheets Average atomic mass Average atomic mass Sreekanthkr Member for 2 years 10 months Age 10 17 Level grade 7 Language English en ID 1586671 30 10 2021 Country code AE Country United Arab Emirates School subject Science 1061951