Answer Key Stoichiometry Worksheet Answers This is a comprehensive end of chapter set of practice problems on stoichiometry that covers balancing chemical equations mole ratio calculations limiting reactants and percent yield concepts The links to the corresponding topics are given below The Mole and Molar Mass Molar Calculations Percent Composition and Empirical Formula
Stoichiometry Worksheet 1 Answers 1 Given the following equation 2 C 4H 10 13 O 2 8 CO 2 10 H 2O show what the following molar ratios should be a C 4H 10 O 2 b O 2 CO 2 c O 2 H 2O d C 4H 10 CO 2 e C 4H 10 H 2O 2 Given the following equation 2 KClO 3 2 KCl 3 O 2 a How many moles of O KEY Solutions for the Stoichiometry Practice Worksheet Balancing Equations and Simple Stoichiometry KEY Balance the following equations 1 1 N2 3 F2 2 NF3 2 2 C6H10 17 O2 12 CO2 10 H2O 3 4 5 1 HBr 1 KHCO3 1 H2O 1 KBr 1 CO2 2 GaBr3 3 Na2SO3 1 Ga2 SO3 3 6 NaBr 3 SnO 2 NF3 3 SnF2 1 N2O3
Answer Key Stoichiometry Worksheet Answers
Answer Key Stoichiometry Worksheet Answers
http://www.worksheeto.com/postpic/2012/06/stoichiometry-worksheet-answers_670813.jpg

Stoichiometry Worksheet 2 Chemistry Answer Key Chemistryworksheet
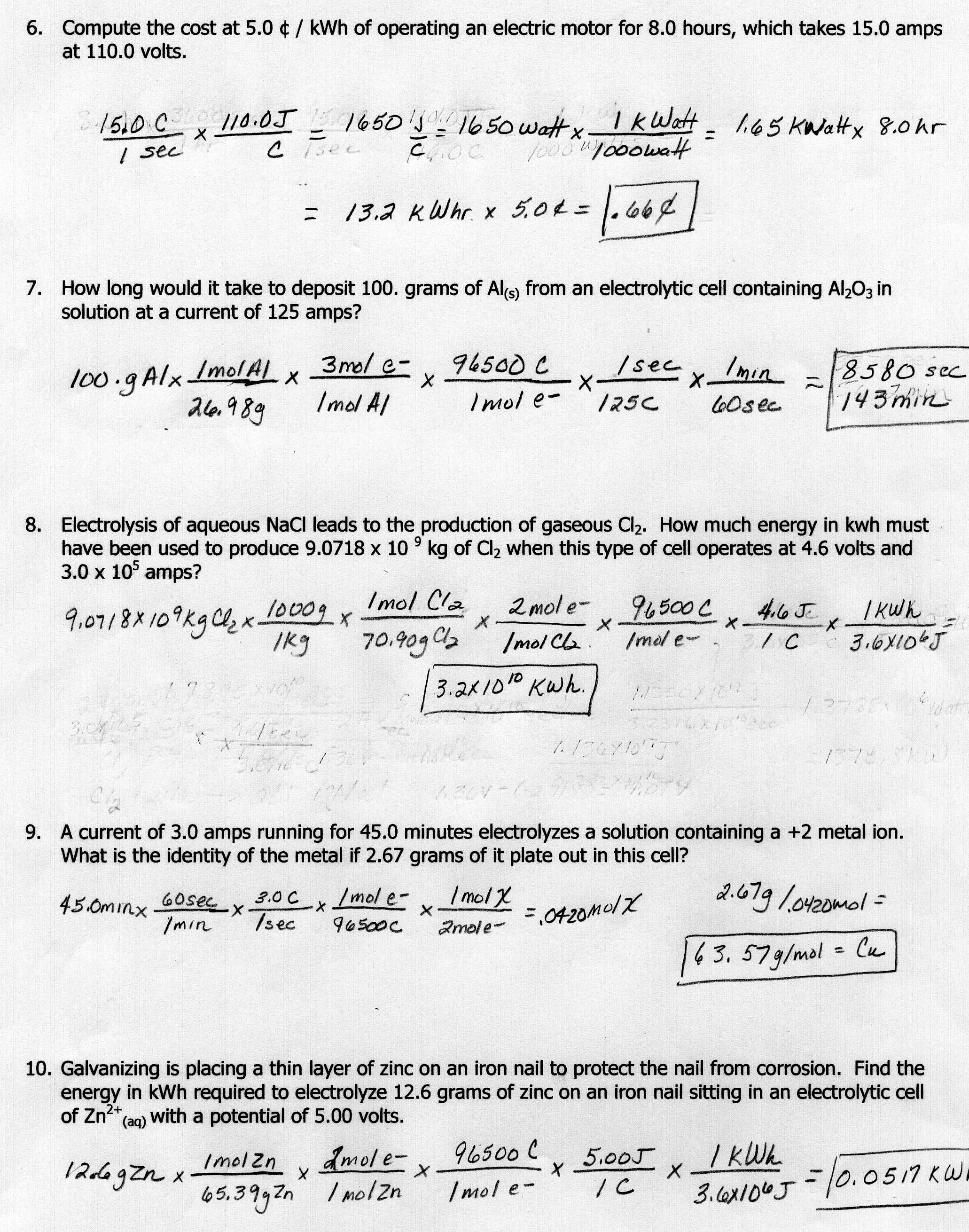
https://www.chemistryworksheet.com/wp-content/uploads/2022/10/stoichiometry-worksheet-2.png
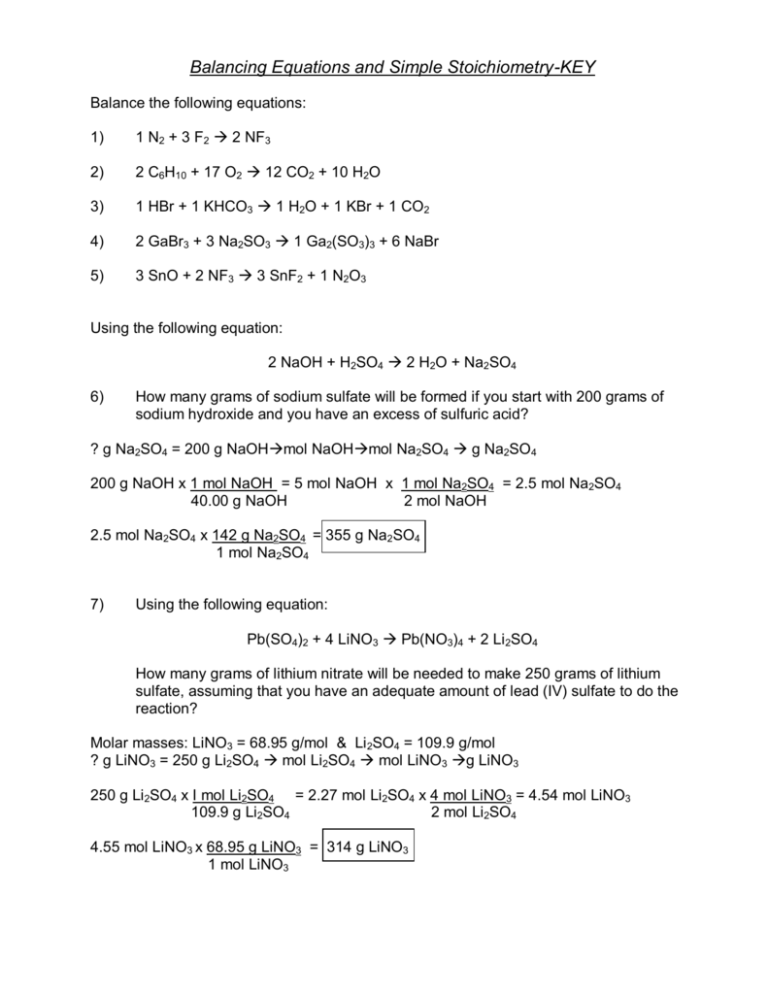
KEY Solutions For The Stoichiometry Practice Worksheet
https://s3.studylib.net/store/data/008711874_1-d12f29b6b62688faeae95fc3bd7c9543-768x994.png
Chapter 12 stoichiometry practice chemistry concepts intermediate answer key chapter 12 stoichiometry 12 1 everyday stoichiometry practice questions use the Skip to document University High School Books Discovery Answers See the answer key at the bottom of the page Review Questions Practice Questions Chemistry Stoichiometry Problem Sheet 2 Directions Solve each of the following problems Show your work including proper units to earn full credit CaCl2 AgNO3 Ca NO3 2 AgCl How many grams of silver chloride are produced when 45 g of calcium chloride react with excess silver nitrate g AgCl 45 g CaCl 2
Q4 Given the following reaction H2SO4 Na2CO3 Na2SO4 H2O CO2 H 2 S O 4 N a 2 C O 3 N a 2 S O 4 H 2 O C O 2 Calculate the molarity of the H2SO4 H 2 S O 4 solution if it takes 40 0 mL of H2SO4 H 2 S O 4 to neutralize 46 7 mL of a 0 364 M Na2CO3 N a 2 C O 3 solution Practice Problems Stoichiometry Answer Key Balance the following chemical reactions a 2 CO O 2 2 CO 2 b 2 KNO 3 2 KNO 2 O 2 c 2 O 3 3 O 2 d NH 4 NO 3 N 2 O 2 H 2 O e 4 CH 3 NH 2 9 O 2 4 CO 2 10 H 2 O 2 N 2 f Cr OH 3 3 HClO 4 Cr ClO 4 3 3 H 2 O Write the balanced chemical equations of each reaction
More picture related to Answer Key Stoichiometry Worksheet Answers

Stoichiometry Problems Worksheet Answers Educational Worksheet
https://s3.studylib.net/store/data/008548006_1-87998e5b1b015fdc34a74176a67dd4ab.png

Chemistry Stoichiometry Worksheet Answers
https://images.sampletemplates.com/wp-content/uploads/2016/11/17162152/Gas-Stoichiometry-Worksheet.jpg
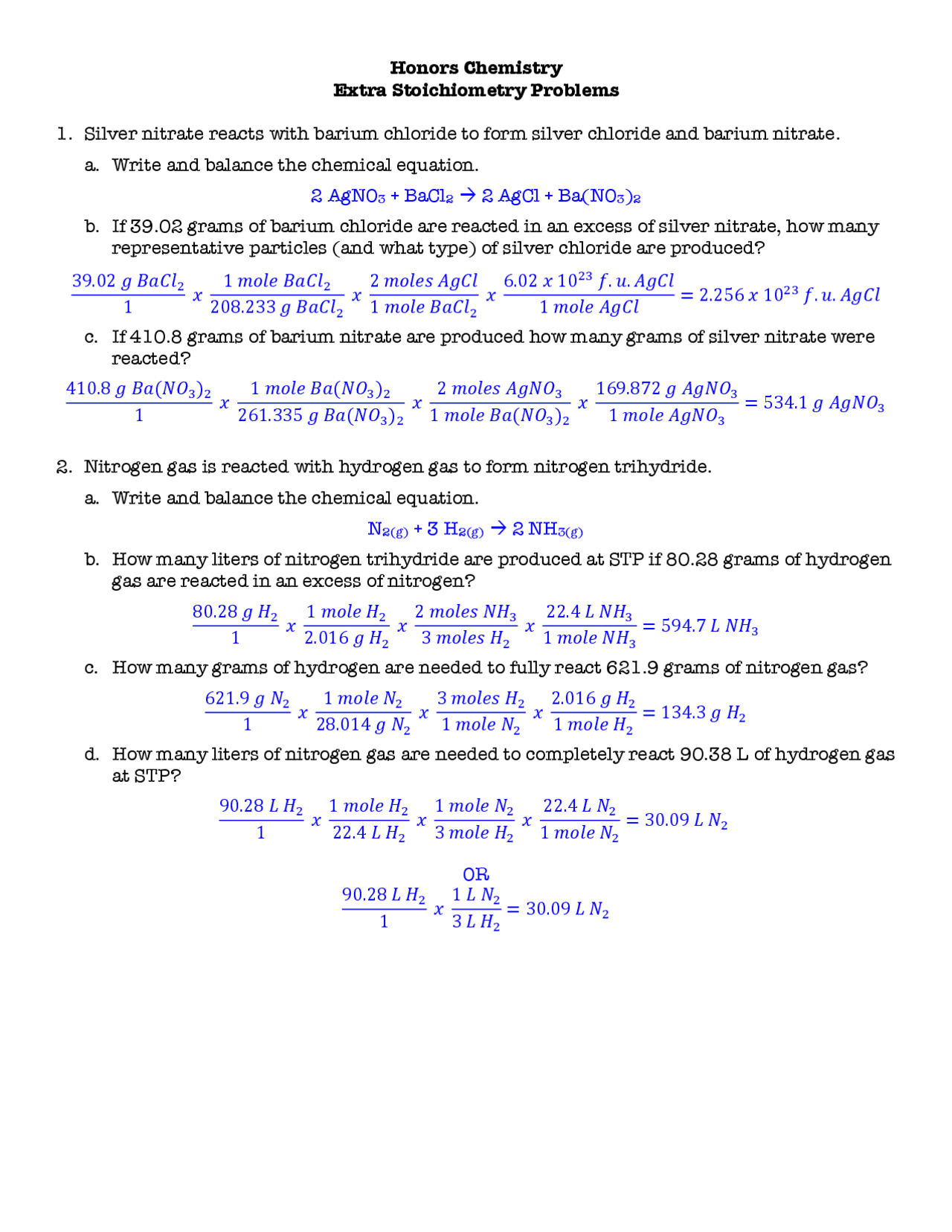
Stoichiometry Worksheet Answer Key
https://static.docsity.com/documents_first_pages/2021/04/20/374813fc83f05cecfbdbe5809326f2b0.png
Chemistry 111 Stoichiometry Worksheet Answer Key Author Shimazu Cheryl Subject Chemistry 111 Stoichiometry Worksheet Answer Key Created Date 2 8 2017 3 51 31 PM Stoichiometry Worksheet 2 Answer Key 1 a 2 13 b 13 8 c 13 10 d 2 8 or 1 4 e 2 10 or 1 5 2 The KClO 3 O 2 molar ratio is 2 3
Solution Stoichiometry Worksheet Solve the following solutions Stoichiometry problems 1 How many grams of silver chromate will precipitate when 150 mL of 0 500 M silver nitrate are added to 100 mL of 0 400 M potassium chromate 2 AgNO3 aq K2CrO4 aq Ag2CrO4 s 2 KNO3 aq 12 4 g Ag2CrO4 13 3 g Ag2CrO4 2 2 KClO 3 2 KCl 3 O 2 How many moles of O 2 will be formed from 1 moles of KClO 3 How many moles of KClO 3 are needed to make 3 moles of KCl How many moles of KCl will be formed from 2 moles of KClO 3 How many moles of Fe 2 are produced when 0 moles of Fe is reacted How many moles of Fe 2 are produced when 31 moles of O 2
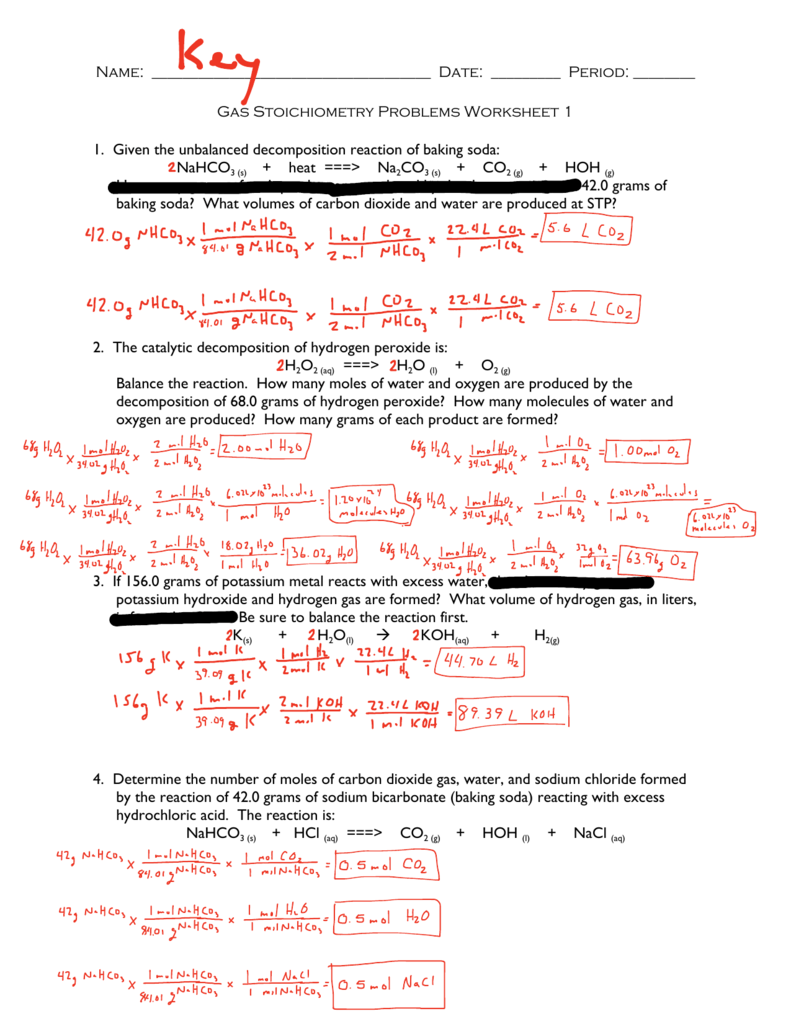
Gas Stoichiometry Worksheet Answer Key
https://s3.studylib.net/store/data/008351424_1-9324fb6e101b050c986aab55e4ce5412.png

Mole Mole Stoichiometry Worksheet Answers
https://s3.studylib.net/store/data/007734847_2-ccaf4ade60b8a5a6d3eb03b9a1dd62e3.png
Answer Key Stoichiometry Worksheet Answers - Answer PROBLEM 5 2 1 5 5 2 1 5 Carborundum is silicon carbide SiC a very hard material used as an abrasive on sandpaper and in other applications It is prepared by the reaction of pure sand SiO 2 with carbon at high temperature Carbon monoxide CO is the other product of this reaction